Thermal Physics: Specific Heat Capacity Explained
advertisement

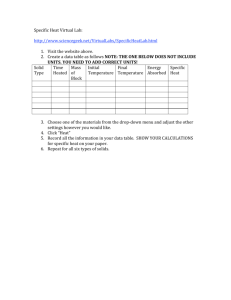
Topic 3 – Lessons 3 and 4 Thermal physics Lesson Outcomes • State the equation for specific heat capacity. GRADE D • Use the equation for specific heat capacity to calculate energy transferred. GRADE C • Explain what it means for a material to have a high specific heat capacity. GRADE B Imagine if…….. Two beakers containing different amounts of water were heated by identical heaters for an equal amount of time. Imagine if…….. The beaker with less water gets hotter. WHY? Imagine if…….. Two beakers, one containing water and one containing kerosene (equal masses) were heated by identical heaters for an equal amount of time. The beaker containing the kerosene would be hotter! WHY? Since the amount of heat energy supplied is the same to both substances, it seems that different substances require different amounts of heat energy to cause the same temperature rise. Definition to learn • Thermal capacity is the amount of energy needed to raise the temperature of a substance/object by 1K. Why are thermal capacities different? • When a substance is heated, its internal energy increases (potential and kinetic). The stronger the force between the particles in the substance, the more heat energy goes into potential energy (and less into kinetic), so the temperature rise is less than in substances with little force between particles. Obviously the more particles there are too, the more heat energy can be absorbed. Calculations using Thermal capacity Energy absorbed = Thermal capacity x Temp rise J J.°C-1 NOT in the data booklet! Q = CΔT °C Specific heat capacity Specific heat capacity is the amount of energy needed to raise the temperature of unit mass of a substance by 1K Specific heat capacity of water = 4186 J.kg-1.°C-1 Specific heat capacity of kerosene = 2010 J.kg-1.°C-1 Specific heat capacity of mercury = 140 J.kg-1.°C-1 Specific Heat Capacity The specific heat capacity of a material is the amount of energy needed to raise the temperature of 1kg of the material by 1°C. Units J/kg°C. SPECIFIC HEAT CAPACITIES Air (typical room conditions) 1012 Lead 129 Aluminium 897 Mercury 139.5 Carbon dioxide 839 Methane 2191 Chromium 449 Nitrogen 1040 Copper 385 Neon 1030.1 Diamond 509.1 Oxygen 918 Ethanol 2440 Paraffin wax 2500 Gasoline 2220 Polyethylene 2302.7 Glass 840 Gold 129 Silica 703 Granite 790 Water at 100 °C (steam) 2080 Graphite 710 Water at 25 °C 4181.3 Helium 5193.2 Water at −10 °C (ice) 2050 Hydrogen 14300 Zinc 387 Iron 450 Calculations using S.H.C. Energy absorbed = Mass x Specific Heat capacity x Temp rise J kg J.kg-1.K-1 Q = mcΔT K Q = m∆t m= c= ∆t= For example 500 g of olive oil is heated until its temperature rises by 120 K. If the specific heat capacity of olive oil is 1970 J.kg-1.K-1, how much heat energy was used? Energy absorbed = Mass x Specific Heat capacity x Temp rise Energy absorbed = 0.5 x 1970 x 120 Energy absorbed = 118200 J A kettle contains 1.5kg of water at a temperature of 18ºC. How much energy is needed to bring the water to the boil? specific heat capacity of water is 4200J/kgºC Water has a very high specific heat capacity – this is why it is used in radiators. The higher the specific heat capacity – the more energy the substance can store. 50kJ of energy was transferred to a material with a mass of 5kg. The temperature increased from 20ºC to 60ºC. What is the specific heat capacity of the material? An analogy: Water and wetness “This analogy is one of my ideas!” Richard Feynmann – Nobel prize winning Physicist, lock-picker and bongo player Two towels – same size/mass • You can add the same amount of water (heat), but the cheaper towel will be “wetter” (temperature). They have different capacities for absorbing water Let’s try some questions! Investigation time! Let’s do an experiment to measure specific heat capacities Specific heat capacities • Cwater = 4181.3 +/- 0.1 J.kg.°C-1 • Caluminium = 897 +/- 1 J.kg.°C-1 Things to note: • If specific heat capacity is constant, the temperature will rise at a uniform rate so long as the power input is constant and no energy is lost to the outside. • There are large potential heat losses if the substance is not well insulated. These can be accounted for in some experiments (how?). • You should be able to think of a number of reasons why your value does not match that in the data book.