Writing Simple Chemical Equations
advertisement

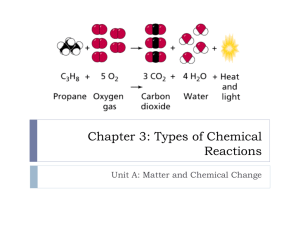
Writing Simple Chemical Equations Balancing and Classification of Reactions Chemical Reactions • Balanced chemical equations represent chemical reactions • A substance which undergoes a chemical change has taken part in a chemical reaction • Remember the differences between chemical and physical change? Chemical Change Physical Change Burning Melting Rusting Freezing Signs of a Chemical Reaction Indicators of a probable chemical reaction – These may be present in some physical changes too 1. Color Changes 2. Precipitation of a solid from a solution 3. Energy changes, heat or light absorbed or released 4. Odor Changes; like baked bread 5. Gas release- sometimes occurs in physical change too Mini-Lab 6.1 Energy Change Question: How can we observe energy changes? Background Information: • All chemical reactions involve an energy change. • Sometimes the change is so small that special detection instruments are needed to observe it. • Sometimes they can be observed easily. • Light, heat, sound, kinetic and potential are all forms of energy. Mini-Lab 6.1 Energy Change Procedure: 1. Place 25g of iron powder and 1g of NaCl in a reseal able bag 2. Add 30g of vermiculite to bag, seal bag and shake 3. Open bag and add 5ml of water, reseal and shake gently 4. Hold bag in your hands, note any changes you observe Mini-Lab 6.1 Energy Change Analysis: (answer the following in your notebook 1. Did you observe energy changes? If so, describe them 2. Did a chemical reaction take place? What kind of reaction? 3. Can you think of anytime in the real world, where this reaction might be useful? Explain Exothermic Reaction The Reaction has as one of the products, energy, in the form of heat! Endothermic Reactions These reactions use energy from the surroundings, in the form of heat, as one of the reactants; creating a cold product! Chemical Equations 1. Describe all changes • Reactants: Iron and that take place Oxygen • Product: Iron(III) Oxide 2. Identify the Rust substances that react a. Called Reactants 3. Identify the substances formed a. Called Products The Description of Changes • More than what can be observed • Ex. “bubbles are formed”, does not tell what gas is formed • A Chemical Equation is how we represent the changes taking place in a reaction • Word equations are the simplest way to describe reactants and products • An arrow, between reactants and products, represents change • + signs are used to separate reactants and to separate products Word Equations • Example: The combining of Vinegar (acetic acid) and Baking soda results in a vigorous reaction, forming a bubbly product The word equation: Vinegar + Baking Soda Sodium acetate + water + Carbon Dioxide Chemical Equations • Shorter than word equations • more exact and specific • Replace the names of the substances with their chemical formulas Example: (the previous reaction) HC2H3O2 + NaHCO3 NaC2H3O2 + H2O + CO2 (Vinegar) Acetic acid Sodium Hydrogen Carbonate Sodium Acetate water Carbon dioxide • By examining the equation you can determine EXACTLY what elements are involved and how many atoms of each Writing Basic Chemical Equations 1. Replace the names of the substances with their chemical formulas – (remember; use oxidation numbers to form stable octets and write compound formulas) 2. Write the chemical equation with all reactants on one side, separated by a (+) sign 3. Draw an arrow pointing toward the products , also separated by a (+) sign. (recall diatomic elements) 4. Add symbols next to each compound, indicating physical state of matter (solid-s, liquid-l, gas-g) Write Chemical Equations; Practice 1. Sodium metal + chlorine gas sodium chloride crystals Answer: Na (s) + Cl2 (g) NaCl(s) Reactants Product Write Chemical Equations; Practice 2. Propane gas + oxygen carbon dioxide + water + energy Answer: C3H8(g) + O2(g) CO2 +H2O(g) + energy Write Chemical Equations; Practice 3. Zinc metal + hydrochloric acid zinc chloride solution + hydrogen Answer: Zn(s) + HCl(aq) ZnCl2(aq) +H2(g) Additional Practice • • • • Hand out #’s 26-30 - with partner (10 min) Post answers! Handout #’s 31-35 – with partner (8 min) Post answers! • Exit Ticket on your own! • Complete #36-50 for homework! Balancing Chemical Equations What is Balancing Equations? • Ensuring every atom in the reactants is present in the products in the same number and type! Why? Law of Conservation of Matter: matter is neither created or destroyed, just transformed from one form to another No creation, no destruction, just rearrangement! Same Number and Type of Atoms Balancing Chemical Equations Steps of process: 1. Write the chemical equation with all reactants on one side, separated by a (+) sign 2. Draw an arrow pointing toward the products , also separated by a (+) sign. (recall diatomic elements) 3. Add symbols next to each compound, indicating physical state of matter (solid-s, liquid-l, gas-g) 4. Count the numbers of each type of element from each side of the equation. 5. List the symbols and numbers of each. 6. Change the coefficients as needed to ensure conservation of matter. (numbers and types equal) Example of Process • Chemical equation for aqueous magnesium chloride plus silver nitrate solution making aqueous magnesium nitrate and solid silver chloride: • Add symbols for physical state • MgCl2(aq) + AgNO3(aq) Mg(NO3)2 (aq) + AgCl(s) • Count number and type of elements on each side Atoms in Reactants Atoms in Products Mg- 1 Mg- 1 Cl- 2 Cl- 1 Ag- 1 Ag- 1 N-1 N-2 O- 3 O- 6 Matter is not conserved! Numbers are different! Practice: Count the number of Atoms • Complete HO 1-10 (pairs) (20 min) Last Step Change the coefficients as needed to ensure conservation of matter. MgCl2(aq) + AgNO3(aq) Mg(NO3)2 (aq) + AgCl(s) Atoms in reactants Mg- 1 Cl- 2 Ag- 1 -2 N-1 - 2 O- 3 -6 Atoms in products Mg- 1 Cl- 1 -2 Ag- 1 2 N-2 O- 6 How can I change the coefficients to make then the same? 1. A 2 added before the AgNO3(aq) in the reactants, will balance both N and O 2. Another 2 added before AgCl(s) in the products will balance both the Ag and Cl MgCl2(aq) + 2 AgNO3(aq) Mg(NO3)2 (aq) + 2 AgCl(s) Predicting Oxidation Numbers • The total charge on the ion is known as the Oxidation Number of the atom Some metals have the same oxidation number in all compounds • Group 1 elements , oxidation number = 1+ • Group 2 elements , oxidation number = 2+ • Aluminum, oxidation number = 3+ – Groups 3-12 Many have more than one oxidation # depending on the reaction • Group 13 elements have 3 valence electrons, oxidation # =3+ • Group 14 may have 2+ or 4+ oxidation number • Groups 15, 16 and 17 tend to gain electrons since they are already ½ full – Their oxidation numbers are 3-, 2-, and 1- respectively Write a Balanced Equation for Each of These Reactions 1. Sodium metal + chlorine gas sodium chloride crystals 2. Propane gas + oxygen carbon dioxide + water + energy 3. Zinc metal + hydrochloric acid zinc chloride solution + hydrogen Remember: • use the oxidation numbers to construct octets and determine formula compounds • Use your reference sheets for names of ionic substances and covalent molecules. (check for diatomic molecules) Answers 1. balance equations for ppt #1.docx 2. balance equations for ppt #2.docx 3. balance equations for ppt #3.docx Answers 1Na (s) + Cl2 (g) NaCl(s) Atoms reactants Atoms products • Na – 1 Na – 1 • Cl – 2 Cl - 1 • Add coefficient of 2 to NaCl to balance Cl • Add coefficient of 2 to Na to balance Na 2 Na (s) + Cl2 (g) 2 NaCl(s) Answers Diatomic molecule • 2. C3H8(g) + O2(g) CO2 +H2O(g) + energy • Propane from reference sheet • Atoms reactants Atoms products • C–3 C–13 • H–8 H–28 • O – 2 10 O – 3 6 10 • Add coefficient of 4 to H2O to balance H • Add coefficient of 3 to CO2 in product to balance C • Add coefficient of 5 to O2 in reactants to balance O • C3H8(g) +5 O2(g) 3 CO2 + 4 H2O(g) + energy • Now it is balanced! Matter was conserved! Answers • 3. Zn(s) + HCl(aq) ZnCl2(aq) +H2(g) • Diatomic molecule • Atoms reactants Atoms products • Zn– 1 Zn– 1 • H–12 H–2 • Cl – 1 2 Cl - 2 • Add coefficient of 2 to HCl to balance H and Cl • Zn(s) + 2 HCl(aq) ZnCl2(aq) +H2(g) • Now it is balanced! Matter was conserved! Let’s Use the Internet to Help us Practice! • Using a computer, Your Brain and your Newly Acquired Skills, practice balancing – Complete at least 10 equations from the beginner level, then 10 at the intermediate level. – Then try 2 or three advanced ones! • Track your progress and record the number of trials it took for each equation in your notebook! • Complete an exit ticket, indicating how many tries it took for each equation • Leave your exit ticket on the laptop cart when you have put your computer away properly! More Balancing! • Access the Following Site: • http://education.jlab.org/elementbalancing/index.html 1. At least 10 Beginner equations and 10 Intermediate! Beginner level Number of trials Intermediate level Number of Trials 1 1 2 2 3 3 4 4 Advanced Level 5 5 1 6 6 2 7 7 3 8 8 9 9 10 10 Number of Trials Exit Ticket 1. Complete an exit ticket, indicating how many tries it took for each equation. Name___________________ EXIT TICKET Total number of beginner equations you tried _____ Total number of trials on beginner equations ______ Total number of intermediate equations you tried ______ Total number of trials on intermediate equations ______ How many advanced equations did you try? How well do you understand this process? Not at all, A little, mostly, I got it! 2. Leave your exit ticket in the box AFTER you have put your computer away properly! Other Sites You Can Use To Practice • http://chemistry.csudh.edu/lechelpcs/rxnbala ncingcsn7.html (pretty good, hard but shows solutions after 3 tries) • http://funbasedlearning.com/chemistry/chem Balancer • http://www.sciencegeek.net/Chemistry/taters /EquationBalancing.htm (All at Once) Types Of Reactions • There are 5 major types of chemical reactions 1. Synthesis 2. Decomposition 3. Single displacement 4. Double displacement 5. Combustion • Reactions can be categorized by recognizing patterns which occur. Synthesis • Pattern to recognize synthesis • Whenever two or more substances combine to form a single product, the reaction is synthesis. Decomposition • Pattern to recognize synthesis • Whenever a compound is broken down into two or more simpler substances it is a decomposition reaction. Single-Displacement Reaction • Pattern to recognize single displacement • Whenever one element takes the place of another in a compound, it is a single displacement reaction. Let’s Classify these reactions! 1. 2. 3. 4. 5. 6. 2 Na + Cl2 2NaCl PCl5 PCl3 + Cl2 2Al + Fe2O3 2Fe + Al2O3 2Ag2O 4Ag + O2 Cl2 + 2KBr 2KCl +Br2 CaO + SiO2 CaSiO3 Answers 1. 2 Na + Cl2 2NaCl 2 substances combine so it is synthesis! 2. PCl5 PCl3 + Cl2 A single compound is broken down into two simpler substances- it is decomposition! 3. 2Al + Fe2O3 2Fe + Al2O3 One element takes the place of another, it is single displacement! Answers Continued 4. 2Ag2O 4Ag + O2 Decomposition 5. Cl2 + 2KBr 2KCl +Br2 Single-displacement 6. CaO + SiO2 CaSiO3 Synthesis Double-Displacement Reactions • Pattern to recognize a double-displacement • Whenever the positive ions of two ionic compounds are interchanged, it is a double displacement reaction. • At least one product must be a precipitate of water! Combustion Reaction • Pattern to recognize combustion • Whenever a substance combines rapidly with OXYGEN to form one or more oxides, is a combustion reaction. Let’s Apply what we have learned! • Classify these reactions: 1. PbCl2 + Li2SO4 PbSO4 + 2LiCl 2. CH4 + 202 CO2 + 2H2O 3. C6H1206 + 602 6CO2 + 6H2O 4. BaCl2 + H2SO4 2HCl +BaSO4 Answers 1. PbCl2 + Li2SO4 PbSO4 + 2LiCl Interchanged cations, so Double-displacement 2. CH4 + 202 CO2 + 2H2O substance combines with Oxygen and forms an oxide, so Combustion 3. C6H1206 + 602 6CO2 + 6H2O Combustion 4. BaCl2 + H2SO4 2HCl +BaSO4 Doubledisplacement Compare and Contrast Types Of Reactions Reaction Type Synthesis General Equation Element/compound + Element/compound compound Examples: 2 Na + Cl2 2NaCl CaO + SiO2 CaSiO3 Decomposition Compound two or more elements/compounds Examples: PCl5 PCl3 + Cl2 2Ag2O 4Ag + O2 Single Displacement Element a + compound bc element b + compound ac Example: 2Al + Fe2O3 2Fe + Al2O3 Cl2 + 2KBr 2KCl +Br2 Double Displacement Compound ac + compound bd compound ad + compound bc Example: PbCl2 + Li2SO4 PbSO4 + 2LiCl BaCl2 + H2SO4 2HCl +BaSO4 Combustion Element/Compound + Oxygen Oxide Example: CH4 + 202 CO2 + 2H2O C6H1206 + 602 6CO2 + 6H2O Identify these Reactions as one of the five types studied 1. 2. 3. 4. 5. 6. 7. 8. A + BX AX +B AB A+B AXZ AX + Z AB + CD AC + BD BC + D BD + C BD + oxygen B oxide + D + water AD + XY AY + DX + + Answers 1. 2. 3. 4. 5. 6. 7. 8. Single displacement (AKA single replacement) Synthesis Decomposition Double Displacement (AKA Double replacement) Single Displacement (AKA Single replacement) Combustion Double Displacement (AKA Double replacement) Decomposition Factors that Affect Direction Of Reactions • External factors modify the direction of reactions • Many can change direction: Called Reversible – Like charging and draining a battery • Adding or removing energy as heat can affect direction – Endothermic reactions; added heat pushes reaction to the right – Exothermic reactions; added heat pushes the reaction to the left Reversibility of Reactions • Not all reactions are reversible – Like fuel burns, food is digested and paint hardens – New products are formed and at least one reactant is used up • Some reactions automatically reverse to establish equilibrium – No net (overall) change – Reactants and products change place, forming at about the same rate – Reactants are never used up because they are always being used then reformed from products – Ex. CaCo3 CaO + CO2 Initiating a Reaction • For a reaction to occur, particles must collide with sufficient force to cause electrons to rearrange. • The amount of energy needed to cause a reaction is called Activation Energy – Slow reactions have high activation energy • To determine reaction rate, measure how quickly one reactant disappears or one product appears Reaction Potential Energy Diagram Shows the potential energy changes that occur as reactants become products. It has five distinct regions: 1. 2. 3. 4. 5. the potential energy of the reactants the potential energy gain that must take place in order for old bonds to be stretched to the breaking point the potential energy of the transition state the potential energy released as new bonds form during a chemical change the potential energy of the products. Details 1. The flat region labeled "Reactants" shows the potential energy of the reacting particles relative to the products. • The actual potential energy of the reactants is an unknown 2. Moving particles possess kinetic energy. When they collide, their kinetic energy is converted to potential energy. 3. The rising part of the graph represents the increase in potential energy that occurs when reactants collide. 4. The minimum gain in potential energy that results in the stretching of reactant bonds to the breaking point is called the activation energy (Ea). It can be determined by experiment. Details 1. 2. The top of the curve represents the point at which the bonds of the colliding particles are stretched to the breaking point. The unstable group of atoms formed at this point are neither reactants nor products but something in between - a transitional structure called the activated complex. (#3) 1. The potential energy of this structure is very high because the bonds are stretched as far as possible. 2. This structure exists for the shortest amount of time imaginable. 3. In an instant, the particles either form new bonds to give new products or reform old bonds to give the original reactants. Details 1. The falling part of the curve represents the energy released when new bonds form between particles to make one or more products. 2. The potential energy difference between the reactants and the products is called the heat of reaction (ΔH). 1. It represents the net energy change of the reaction. 3. If the potential energy of the products is greater than that of the reactants, then the reaction is classified as endothermic. 4. If the potential energy of the products is less than that of the reactants, then the reaction is classified as exothermic. Details 1. The second flat region represents the potential energy of the products. The actual potential energy of the products is also an unknown. 2. The difference in energy between reactant and products determines endothermic vs exothermic reactions 1. absorbs energy vs releases energy Important notes! • Reactions with low activation energy are fast, while those with high activation energy are slow. • The higher the activation energy, the slower the rate of the reaction. • If the potential energy of the reactants is greater than that of the products, the reaction is exothermic - it results in the net release of potential energy as heat. • If the potential energy of the reactants is less than that of the products, the reaction is endothermic - it results in the net gain of energy from some external source (e.g. the sun) which is then stored in the products. Factors that Affect Rate Of Reactions 1. Temperature; most reactions speed up with higher temp 2. Concentration; raising the concentration of a reactant can speed up a reaction 3. Catalysts; speeds up rate w/out being changed by the reaction (enzymes in cells) 4. Inhibitor; slows down a reaction (preservatives) Review Problems • What are the correct coefficients of these reactions? 1. 2. 3. 4. 5. • KNO3 + H2CO3 K2CO3 + HNO3 SnO2 + H2 Sn + H2O SeCl6 + O2 Se O2 + Cl2 N2 + H2 NH3 P4 + O2 P2O5 Answer the following; 1. 2. 3. 4. 5. What is activation energy? What happens if there is not enough activation energy? Why do you need a match to start a fire? What are the indicators that a reaction has taken place? What does each of the following mean, when in a chemical equation? a) b) c) d) (g) (s) (l) (aq) Answers to Review Problems 1. 2, 1, 1, 2 2. 1, 2, 1, 2 3. 1, 1, 1, 3 4. 1, 3, 2 5. 1, 5, 2 Answer the following; 1. The amount of energy needed to start a reaction 2. The reaction will not start 3. It provides activation energy 4. Color change, precipitation, energy change, odor or gas production 5. What does each of the following mean a) b) c) d) (g) gas (s) solid (l) liquid (aq) dissolved in water Test Tomorrow! • Study your notes and review Handout! • Practice answering questions, and writing and balancing equations • Prepare by looking through your notes before class! • Visit the wiki for additional help! • Organize your notebook, check your checklist!