Writing Chemical Equations
advertisement

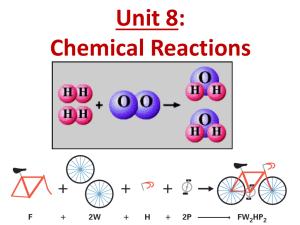
Writing Chemical Equations SCH3U What is a chemical reaction? • When elements or compounds interact and rearrange to form new substances • The substance(s) that undergo the change are called reactants and the substances that are formed in the chemical reaction are called products. reactants products Law of Conservation of Mass • What is this? Matter cannot be created nor destroyed during a chemical reaction. All atoms present in the reactants must be present in the products. Writing Chemical Equations • Three ways to write chemicals equations: • Word Equations • Identify the reactants and products by chemical name and their states (solid, liquid, gas & aqueous) • Eg. Methane gas + oxygen gas carbon dioxide gas + water • Skeleton Equations • Identifies the reactants and products by their chemical formula, including the state of the molecule • Eg. CH4(g) + O2 (g) CO2 (g) + H2O(l) Writing Chemical Equations • Balanced Chemical Equation • Reflects the law of Conservations of Mass and shows that there are the same number of atoms on each side of the reaction equation. • Eg. CH4(g) + 2 O2 (g) CO2 (g) + 2 H2O(l) Steps to Balancing Equations 1. Check to make sure your skeleton equation is written correctly (ie. balance charges in 2. For each element, count the number of atoms on each side of the equation. (If a 3. If the number of atoms are not equal, you need to balance the reactants and products using coefficients. 4. Start with elements other than H & O. 5. Add a coefficient in front of the chemical formula for any unbalanced elements. 6. REMEMBER – you cannot change any of the elements or subscripts in a chemical formula! 7. Count up all the atoms on each side to make sure you are now in balance. ionic compounds, check your Greek prefixes on molecular compounds). polyatomic ion is on both sides of the equation – UNCHANGED, then keep it as one, do not separate it into its ions.) Try This: • Writing Chemical Equations Worksheet