Redox Reactions, Acids, and Bases Chemistry Worksheet
advertisement

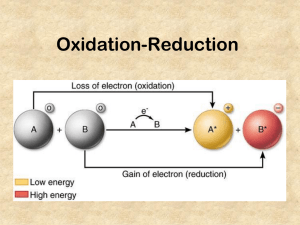
Chemical Reactions, Acids and Bases Chemistry 6. Oxidation-Reduction (Redox) Reactions Electrons are transferred from one atom to another. Note that when one substance is oxidized, another compound must be reduced, and viceversa. Oxidation = - electrons from a substance Reduction = + electrons to a substance Mg(s) + 2 HCl(aq) MgCl2(aq) + H2(g) 0 +1 -1 +2 -1 0 • • • Note: The transfer of electrons during oxidation-reduction reactions often produce energy (when spontaneous), which can be in the form of electricity. LEO the lion says GER (Loss of Electrons = Oxidation/Gain of Electrons = Reduction) OIL RIG (oxidation is loss and reduction is gain) Examples: a) Combustion: “rapid oxidation reaction in which a large amount of heat and usually light are released” C + O2 CO2, CO S + O2 SO2 What was oxidized? What was reduced? b) Metal + acid (SR): e.g. Zn(s) + 2 HCl(aq) ZnCl2(aq) + H2(g) Total ionic equation: What was oxidized? What was reduced? c) Metal + salt (SR): e.g. Mg(s) + CoSO4(aq) MgSO4(aq) + Co(s) Total ionic equation: What was oxidized? What was reduced? Oxidation numbers help us keep track of what happens to the electrons in reactions. -1- Chemical Reactions, Acids and Bases Chemistry Practice Identify redox pairs and oxidation numbers. Write net ionic equations for the following reactions. 2 Na(s) + SrBr2(aq) Sr(s) + 2 NaBr(aq) Zn(s) + H2SO3(aq) ZnSO3(aq) + H2(g) 2 Na(s) + 2 H2O(l) 2 NaOH(aq) + H2(g) Fe(s) + CuCl2(aq) Cu(s) + FeCl2(aq) Predicting Products of Different Reaction Types Class of reaction Synthesis Decomposition Reactants two or more substances one compound Single Replacement a metal and a compound Double Replacement a nonmetal (halogen) and a compound two compounds dissolved in water hydrocarbon + oxygen Combustion (restricted definition) Oxidation-Reduction one or more substances -2- Probable Products