Oxidation-Reduction Reactions: Chemistry Presentation
advertisement
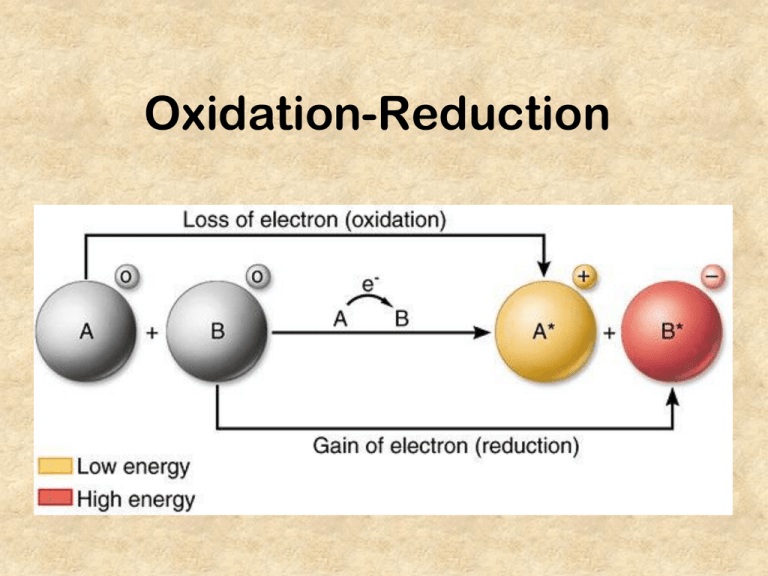
Oxidation-Reduction 1+ 2+ 3+ ...etc. 4+/- 3- 2- 1- 0 Recognize these! NO31- nitrate NO21- nitrite OH1- hydroxide ClO21- chlorite ClO31- chlorate HCO31SO42- hydrogencarbonate (bicarbonate) sulfate SO32- sulfite CO3 2- carbonate PO43- phosphate NH41+ ammonium Introduction • oxidation and reduction can be considered in terms of… 1. oxidation- substance gains oxygen reduction- substance loses oxygen 2. oxidation- the loss of electrons reduction- the gain of electrons 3. oxidation- the oxidation state/# increases reduction- the oxidation state/# decreases O I L - oxidation - is - loss of electrons R I G - reduction - is - gain of electrons Oxidation states Note: oxidation states must be written with the sign in FRONT: +2 not 2+ • the oxidation state (number) is the apparent or theoretical charge of an free element, molecule, or ion • oxidation – a process where the number increases (more positive because loses neg. electrons) • Ex: Mg Mg2+ (aq) + 2e- • reduction – a process where the number decreases (less positive/more negative because gains neg. electrons) • Ex: O + 2 e- O2- (g) Rules for assigning oxidation states 1 The oxidation number of an free element is always 0 O2, H2, Ne, Zn 2 The oxidation number of Hydrogen is usually +1 HCl, H2SO4 3 The oxidation number of Oxygen is usually -2. H2O, NO2, etc. 4 Group 1 metals are always +1 Group 2 metals are always +2 Aluminum is always +3 Li, Na… Mg, Ba.. Al 5 Fluorine is always -1 Other group 17 (halogens) are often -1 HF, OF2 HI, NaCl, KBr 6 Oxidation numbers of ions follow the charge of the ion S2-, Zn2+ 7 The SUM of oxidation numbers is zero for a neutral compound. LiMnO4… 8 For polyatomic ions, the SUM is their charge. SO42-, NO31- Practice Assigning Oxidation Numbers O2 0 N2O5 O is -2 x 5 = -10 N must equal +10/2 = +5 HClO3 (+1) + (x) + 3(-2) = 0; x = (+5) HNO3 O is -2 x 3 = -6; H is +1; N must equal +5 Ca(NO3)2 O is -2 x 3 = -6 x 2 = -12; Ca is +2; N must equal +10/2 = +5 KMnO4 O is -2 x 4 = -8; K is +1; Mn must equal +7 Fe(OH)3 O is -2 x 3 = -6; Fe is +3; H is +1 x 3 = +3 K2Cr2O7 O is -2 x 7 = -14; K is +1 x 2 = +2; Cr must equal +12/2 = +6 CO32- x + 3(-2) = -2 x = +4 CN- N is -3; Charge = -1 means that C must be +2 K3Fe(CN)6 N is -3 x 6 = -18; C is +2 x 6 = +12; K is +1 x 3 =+3; Fe must be +3 CH4 H is +1 x 4 = +4; C must be -4 Using Oxidation Numbers • an increase in the oxidation number (more +) indicates that an atom has lost electrons and therefore is oxidized • a decrease in the oxidation number (less +) indicates that an atom has gained electrons and therefore reduced • Example Zn + CuSO4 ZnSO4 + Cu 0 +2 +6-2 +2+6-2 0 Zn: 0 + 2 Oxidized Cu: +2 0 Reduced S and O are unchanged NO31NO21OH1- ClO21ClO31- HCO31- SO42SO32- CO3 2PO43- NH41+ Recogniz Exercise For each of the following reactions (not balanced to simplify) find the element oxidized and the element reduced Cl2 Cu + KBr KCl + Br2 + HNO3 Cu(NO3)2 + NO2 + H2O HNO3 + I2 HIO3 + NO2 Exercise For each of the following reactions find the element oxidized and the element reduced Cl2 0 + KBr +1-1 KCl + +1-1 Br loses an electron -- oxidized Cl gains an electron -- reduced K remains unchanged at +1 Br2 0 Exercise For each of the following reactions find the element oxidized and the element reduced Cu + HNO3 Cu(NO3)2 + NO2 + H2O 0 +1+5-2 +2 +5 -2 +4 –2 +1 -2 • Cu increases from 0 to +2. It is oxidized • Only part of the N in nitric acid changes from +5 to +4. It is reduced • The nitrogen that ends up in copper nitrate remains unchanged Recognize these! NO31- nitrate NO21- nitrite OH1- hydroxide ClO21- chlorite ClO chlorate 1- Exercise For each of the following reactions find the element oxidized and the element reduced HNO3 + +1+5-2 • • • I2 HIO3 + 0 +1+5-2 NO2 +4-2 N is reduced from +5 to +4. It is reduced. I is increased from 0 to +5 It is oxidized The hydrogen and oxygen remain unchanged. Agents • all redox reactions have one element oxidized and one element reduced • the compound that supplies the electrons (is oxidized) is the reducing agent • the compound that gains the electrons (is reduced) is the oxidizing agent Exercise For each of the following reactions find the element oxidized, element reduced, and the agents. HNO3 + +1+5-2 • 0 HIO3 + NO2 +1+5-2 +4-2 N is reduced from +5 to +4. – • I is the oxidizing agent that caused this I is oxidized from 0 to +5. – • I2 N is the reducing agent that caused this The hydrogen and oxygen remain unchanged.







