Day 25 – Carbohydrates
advertisement

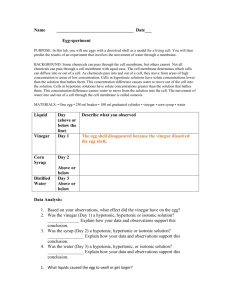
Happy Tuesday Bellwork: Quickwrite – In 42 words, summarize your HW using the words concentration, diffusion, osmosis, equilibrium, isotonic, hypertonic, and hypotonic. On Page 17 of your I.A.N. Title “Egg Lab” and draw the following data table: Original Mass VINEGAR SYRUP WATER XXXXXXXXX Final Mass Difference in Mass XXXXXXXXX Appearance of Egg On Page 17 of your I.A.N. Title “Egg Lab” and draw the following data table: Original Mass VINEGAR XXXXXXXXX Final Mass Difference in Mass Appearance of Egg XXXXXXXXX SYRUP WATER The shell that surrounded your egg was dissolved by soaking the egg in vinegar for 24 hours. The vinegar dissolved the hard calcium carbonate shell exposing the semi-permeable membrane that surrounds the egg. This membrane is very similar to the plasma membrane that surrounds all of our cells. The purpose of this lab is to simulate osmosis across a plasma membrane. Students will receive one egg per lab table. Day 1 Procedure… 1. Using your hands, carefully remove your egg from the vinegar and use a paper towel to pat it dry. 2. On your data table, record the firmness and overall appearance of your egg in the VINEGAR row. 3. Mass (weigh) your egg on a triple beam balance and record your results on your data table. Enter the weight under the “Final Mass” for VINEGAR and the “Original Mass” for SYRUP. 4. Using a piece of glassware tape and a marker, label the beaker with your period and table letter. Cover the egg with syrup until it begins to float. 5. Allow your egg to sit for 24 hours. Diffusion Notes Why does food coloring spread out in water? Every living thing lives in a liquid environment that it needs to survive. One of the most important functions of the cell membrane is to regulate the movement of dissolved molecules from the liquid on one side of the membrane to the liquid on the other side. The concentration is the amount of a substance dissolved into water. Kool-Aid Beaker 1 Beaker 2 Beaker 3 In a solution, particles are constantly moving. They collide with one another and tend to spread out randomly. Particles move from an area of high concentration to an area of low concentration. This process is known as diffusion. A concentration gradient is a difference between two concentrations. Diffusion is the movement of particles down the concentration gradient. When the concentration of a solute is the same throughout a system, the system has reached equilibrium. When equilibrium is reached, particles do continue to move across the membrane. Substances that diffuse across a membrane do not require the cell to use energy. If a substance is able to diffuse across a membrane, the membrane is said to be permeable to it. If a substance cannot diffuse across a membrane, the membrane is said to be impermeable to it. Selectively permeable means that some substances can pass across while others cannot. Biological membranes are selectively permeable. Diffusion of water through a selectively permeable membrane is called osmosis. When two solutions have the same concentration of solute they are said to be isotonic. Isotonic means “same strength”. Draw This A solution that has a higher concentration of solute is said to be hypertonic. Hypertonic means “above strength”. Draw This A solution that has a lower concentration of solute is said to be hypotonic. Hypotonic means “below strength”. Draw This