Measuring and Significant Figures Powerpoint
advertisement

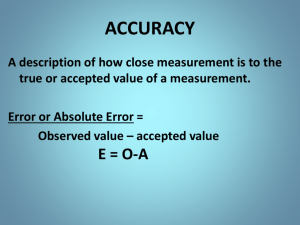
Measuring Keeping track of precision Two Types of numbers • The two types are exact and measured. • Exact numbers are arrived at through counting. Example: How many students are in this class? • Measured numbers are found by using a measuring instrument (ruler, bathroom scale) and estimating the readout of the instrument. All measurements are estimates! • We may know some parts of a measurement with certainty but even digital readouts are typically accurate to within + or – 5% of the measured value. Accuracy vs. precision • If we measure something properly then our measurement is accurate and reflects what we are actually measuring. • Precision is indicated by how many significant digits we need to describe a measurement. Compare 100 km/h to 102.35 km/h. Which is more precise? • It is our goal in physics to be as accurate and as precise as possible. Significant Digits • The digits 1, 2, 3, 4, 5, 6, 7, 8, 9, are always significant (125 has 3 sig digits) Zeros • Zeros are not significant when only used as placeholders (100 and 0.01 each have 1 significant digit) • Zeros are significant when – Sandwiched between two significant digits • (105 has 3 sd, so does 1050) – Found to the right of a decimal and not used as a placeholder ( 0.050 and 5.0 each have 2 significant digits. Check yourself • How many Significant digits do each of the following numbers have? 1236 2500 2500.0 0.00510 2500.00510 Important points • The precision of a number is indicated by the number of significant digits in the number. • Whether or not a digit is significant can be determined by whether or not the digit adds precision to the number. Scientific Notation • Only the digits in the first factor are significant. (The 2nd factor is a power of ten which is the “placeholder” for the number). Calculations • When measured values become the numbers used for a calculation the end result of the calculation has the same degree of precision as the least precise number that went into the calculation. (A chain is as strong as its weakest link) There are many rules governing how many sig figs an answer should have based on the precision of the input values… We will ignore these. • The rules we will use in class are as follows: 1. All final answers should have 3 + or – 1 Significant digits. 2. All intermediate values used in a calculation should have a minimum of 5 significant digits. For Practice • Significant figures practice worksheet with answers go to: http://misterguch.brinkster.net/PRA006.pdf • For Rounding rules go to the lab manual found on the grade 11 section Of Mr. Turton’s website page 12: http://hrsbstaff.ednet.ns.ca/max/Grade%2 011%20Documents/2008%20Fall%20Phy sics%2011%20Lab%20Manual.pdf Precision in the lab • Precision of measured values is typically indicated with absolute uncertainty . • Precision of final results is typically indicated with a percent uncertainty. Absolute uncertainty • Example: 50.5 ± 0.2 cm • 50.5 cm is the best estimate of the measurement. • 0.2 cm is the uncertainty in the measurement. • This means that the measurement could reasonably be as low as 50.3 cm or as high as 50.7 cm. Percent Uncertainty • Example: The absolute uncertainty in 50.5 ± 0.2 cm can be converted into a percent uncertainty as follows • 0.2/50.5 = 0.003960 • 0.003960 x 100 = 0.396% = 0.4% • Percent uncertainty : 50.5 cm ± 0.4% • Both types of uncertainty are represented with one significant figure. Uncertainty agreement • 50.536 ± 0.2 cm is unreasonable – Why would we be concerned with the extra 0.036 cm when using our best judgement the measurement could be off by as much as 0.2 cm ? – The measured or calculated value should agree with the stated uncertainty. • 50.5 ± 0.2 cm shows this agreement Uncertainty from Sig Figs • A number expressed with the appropriate number of significant figures may be converted into an absolute uncertainty as ± 2 of the most precise digit in the measurement • 105 km/h = 105 ± 2 km/h • 1030 m = 1030 ± 20 m • 0.105 kg = 0.105 ± 0.002 kg A measurement made without any knowledge of its precision is meaningless. That’s all folks.