SPH3U Measurement and Uncertainty
advertisement
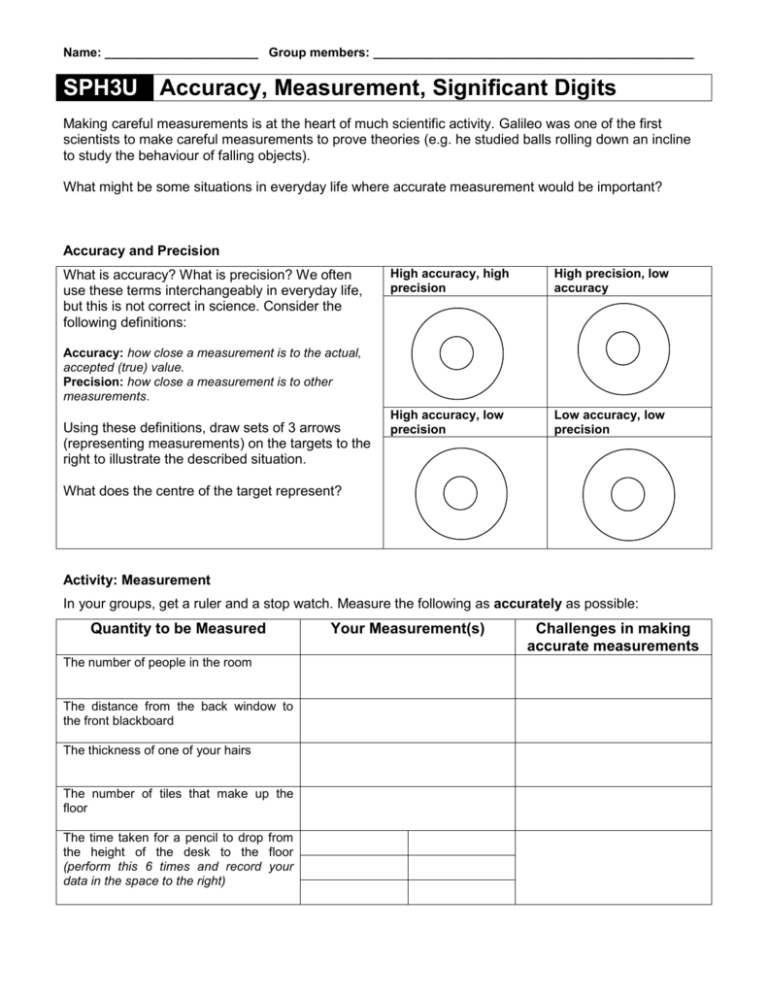
Name: ______________________ Group members: ______________________________________________ SPH3U Accuracy, Measurement, Significant Digits Making careful measurements is at the heart of much scientific activity. Galileo was one of the first scientists to make careful measurements to prove theories (e.g. he studied balls rolling down an incline to study the behaviour of falling objects). What might be some situations in everyday life where accurate measurement would be important? Accuracy and Precision What is accuracy? What is precision? We often use these terms interchangeably in everyday life, but this is not correct in science. Consider the following definitions: High accuracy, high precision High precision, low accuracy High accuracy, low precision Low accuracy, low precision Accuracy: how close a measurement is to the actual, accepted (true) value. Precision: how close a measurement is to other measurements. Using these definitions, draw sets of 3 arrows (representing measurements) on the targets to the right to illustrate the described situation. What does the centre of the target represent? Activity: Measurement In your groups, get a ruler and a stop watch. Measure the following as accurately as possible: Quantity to be Measured The number of people in the room The distance from the back window to the front blackboard The thickness of one of your hairs The number of tiles that make up the floor The time taken for a pencil to drop from the height of the desk to the floor (perform this 6 times and record your data in the space to the right) Your Measurement(s) Challenges in making accurate measurements Discussion 1. For a given quantity, we may obtain a range of measurements. What do we do in this case and why? 2. For the pencil drop activity, our repeated measurements produced a range of values; i.e. our measurements have uncertainty in them. What factors might account for the uncertainty of your measurements in this activity? Which of these factors probably affect the accuracy of your measurement the most? 3. What might we do to reduce the uncertainty in our pencil drop measurements? Why can we not completely eliminate all uncertainty in our measurements? In your groups, read and discuss: Uncertainty and Significant Digits (Sig. Digs.) In the past, you may have learned some rules regarding significant digits (sig. digs.). The purpose of these rules was to help you express the amount of uncertainty in a calculation involving measured values. Example 1: a measurement of 12.3 m (3 sig. digs.) indicates that the 12 m is certain but that there is uncertainty in the “.3” (the actual value might be 12.2 m or 12.5 m). How many significant digits does 2598 nanoseconds have, and what does it mean? Example 2: What is the difference between stating a measurement is 12.3 m (3 sig. digs.) and stating it as 12.30 m (4 sig. digs.)? In real life, we usually report a calculated result based on measurements as 2 numbers: the best or average number (m), and the uncertainty (σ – Greek letter sigma), written as m ± σ. This is interpreted as saying that “m is our best estimation, but the true value could be as high as m + σ, or as low as m – σ.” Example 3: If the average height of the class is reported as 165 ± 12 cm, how tall (or short) are the tallest and shortest members of the class likely to be? This year, we will simplify how we report results or textbook calculations. (We will do more with uncertainty in grade 12 physics): When recording results, just use 3 sig. digs. in scientific notation to avoid rounding error. e.g. your calculator reads: 23 162, you write 2.32 × 104 For middle steps in calculations, keep 1 or 2 extra digits to help reduce rounding error. For convenience, we will write 5 instead of 5.00 with the understanding that it has 3 sig. digs.