Name Period
advertisement

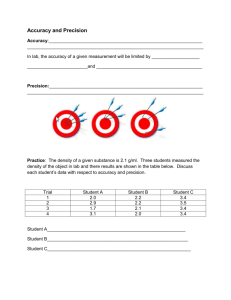
Name ___________________________________ Date _______________ PRECISION, ACCURACY AND SIGNIFICANT DIGITS IB Chemistry HL Juniors 5092-5093 1. Three students each use the same meter stick to measure the length of a room. The actual room length is 11.25 meters and they report the following measurements: Student A’s measurement: Student B’s measurement: Student C’s measurement: 10.94 meters 10.93 meters 10.93 meters A. Describe the precision. __________________________________________________ B. Describe the accuracy. __________________________________________________ C. How many Sig Figs in each measurement? __________________________________ D. How many certain digits in each measurement? ______________________________ 2. The boiling point of pure water at sea level is known to be 100.0ºC. Using a new thermometer, a student measures the boiling point of pure water (at se level) three times. Each time the thermometer reads 98.6ºC. A. Describe the precision. __________________________________________________ B. Describe the accuracy. __________________________________________________ C. How many Sig Figs in each measurement? __________________________________ D. What is the percent error? _______________________________________________ 3. A standard 20.00 g mass is used to check the accuracy of a laboratory balance. The balance indicates a mass of 19.81 g on each of 4 measurements when the standard mass is measured. A. Describe the accuracy of the balance. _______________________________________ B. Describe the precision of the balance. _______________________________________ C. What is the percent error of this measurement? ________________________________ 4. Five students each weigh the same object on the same balance. The actual mass of the object is known to be 3.135 g. Their results are: Student 1: 3.137 g Student 4: 2.832 g Student 2: 3.137 g Student 5: 3.136 g Student 3: 3.134 g A. Describe the precision of the balance. _______________________________________ B. Describe the accuracy of the balance. _______________________________________ C. Comment on the 5 students: _______________________________________________ D. How many sig figs are in these values? ______________________________________ 5. How many significant digits are in the following? a. 0.0789 m __________ d. 2090 cm __________ b. 200010 g __________ e. 49.00 L ___________ c. 105.30 mL _________ f. 300 cm3 ___________ 6. Calculate the following using correct significant digits: a. 27.40 – 3.7 d. (4.75 – 3.89)/4.75 b. 300 + 2.5 e. 200.0 x 28 x 4.18 c. 8.69 – 4.29 f. (252 x 3.1) / 80 7. Convert the following metric values: a. 278 mm to km d. 24 Mg to cg b. 23.5 hL to dL e. 374 mg to μg c. 5.89 nm to dam f. 49.6 nm to μm