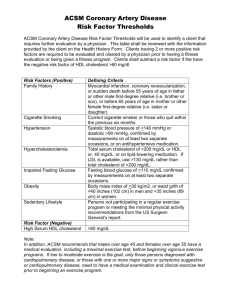
8- Chapter Summary
advertisement

UNIT III: Lipid Metabolism Cholesterol and Steroid Metabolism E. Metabolism of HDL HDL comprise a heterogeneous family of lipoproteins with a complex metabolism that is not yet completely understood. HDL particles are formed in blood by the addition of lipid to apo A-1, an apolipoprotein made by the liver and intestine and secreted into blood. Apo A-1 accounts for about 70% of the apoproteins in HDL. HDL perform a number of important functions, including the following: 1. HDL is a reservoir of apolipoproteins: HDL particles serve as a circulating reservoir of: apo C-II (the apolipoprotein that is transferred to VLDL and chylomicrons, and is an activator of lipoprotein lipase), and apo E (the apolipoprotein required for the receptor-mediated endocytosis of IDLs and chylomicron remnants). E. Metabolism of HDL 2. HDL uptake of unesterified cholesterol: Nascent HDL are disk-shaped particles containing primarily phospholipid (largely phosphatidylcholine) and apolipoproteins A, C, and E. They are rapidly converted to spherical particles as they accumulate cholesterol (Figure 18.23). Note: HDL particles are excellent acceptors of unesterified cholesterol (both from other lipoproteins particles and from cell membranes) as a result of their high concentration of phospholipids, which are important solubilizers of cholesterol. E. Metabolism of HDL 3. Esterification of cholesterol: When cholesterol is taken up by HDL, it is immediately esterified by the plasma enzyme phosphatidylcholine:cholesterol acyltransferase (PCAT, also known as LCAT, in which “L” stands for lecithin). This enzyme is synthesized by the liver. PCAT binds to nascent HDL, and is activated by apo A-I. PCAT transfers the fatty acid from carbon 2 of phosphatidylcholine to cholesterol. This produces a hydrophobic cholesteryl ester, which is sequestered in the core of the HDL, and lysophosphatidylcholine, which binds to albumin. As the nascent HDL accumulates cholesteryl esters, it first becomes a relatively cholesteryl ester–poor HDL3 and, eventually, a cholesteryl ester–rich HDL2 particle that carries these esters to the liver. Figure 18.23 Metabolism of HDL. PC = phosphatidylcholine; lyso-PC = lysophosphatidylcholine. PCAT = Phosphatidylcholine cholesterol transferase. CETP = cholesteryl ester transfer protein. ABCA1 = transport protein. [Note: For convenience the size of VLDLs are shown smaller than HDL, whereas VLDLs are larger than HDL.] 5 E. Metabolism of HDL 4. Reverse cholesterol transport: The selective transfer of cholesterol from peripheral cells to HDL, and from HDL to the liver for bile acid synthesis or disposal via the bile, and to steroidogenic cells for hormone synthesis, is a key component of cholesterol homeostasis. This is, in part, the basis for the inverse relationship seen between plasma HDL concentration and atherosclerosis, and for HDL's designation as the “good” cholesterol carrier. Reverse cholesterol transport involves efflux of cholesterol from peripheral cells to HDL, esterification of cholesterol by PCAT, binding of the cholesteryl ester–rich HDL (HDL2) to liver and steroidogenic cells, the selective transfer of the cholesteryl esters into these cells, and the release of lipid-depleted HDL (HDL3). E. Metabolism of HDL The efflux of cholesterol from peripheral cells is mediated, at least in part, by the transport protein, ABCA1. Note: Tangier disease is a very rare deficiency of ABCA1, and is characterized by the virtual absence of HDL particles due to degradation of lipid-poor apo A-1. The uptake of cholesteryl esters by the liver is mediated by a cellsurface receptor, SR-B1 (scavenger receptor class B type 1) that binds HDL (see p, 234 for SR-A). It is not yet clear as to whether the HDL particle itself is taken up, the cholesteryl esters extracted, and the lipid-poor HDL released back into the blood, or if there is selective uptake of the cholesteryl ester alone. Figure 18.23 Metabolism of HDL. PC = phosphatidylcholine; lyso-PC = lysophosphatidylcholine. PCAT = Phosphatidylcholine cholesterol transferase. CETP = cholesteryl ester transfer protein. ABCA1 = transport protein. [Note: For convenience the size of VLDLs are shown smaller than HDL, whereas VLDLs are larger than HDL. 8 E. Metabolism of HDL Note: Hepatic lipase, with its ability to degrade both TAG and phospholipids, participates in the conversion of HDL2 to HDL3 ABCA1 is an ATP-binding cassette (ABC) protein. ABC proteins use energy from ATP hydrolysis to transport materials, including lipids, in and out of cells and across intracellular compartments. In addition to Tangier disease, defects in specific ABC proteins result in X-linked adrenoleukodystrophy, respiratory distress syndrome due to decreased surfactant secretion, and cystic fibrosis. F. Role of lipoprotein (a) in heart disease Lipoprotein (a), or Lp(a), is a particle that, when present in large quantities in the plasma, is associated with an increased risk of coronary heart disease. Lp(a) is nearly identical in structure to an LDL particle. Its distinguishing feature is the presence of an additional apolipoprotein molecule, apo(a), that is covalently linked at a single site to apo B-100. Circulating levels of Lp(a) are determined primarily by genetics. F. Role of lipoprotein (a) in heart disease However, factors such as diet may play some role, as trans fatty acids have been shown to increase Lp(a), and estrogen decreases both LDL and Lp(a). Note: Apo(a) is structurally homologous to plasminogen—the precursor of a blood protease whose target is fibrin, the main protein component of blood clots. It is hypothesized that elevated Lp(a) slows the breakdown of blood clots that trigger heart attacks because it competes with plasminogen for binding to fibrin. 7- Steroid Hormones Cholesterol is the precursor of all classes of steroid hormones: glucocorticoids (for example, cortisol), mineralocorticoids (for example, aldosterone), and sex hormones—androgens, estrogens, and progestins (Figure 18.24). Note: Glucocorticoids and mineralocorticoids are collectively called corticosteroids. Synthesis and secretion occur in the adrenal cortex (cortisol, aldosterone, and androgens), ovaries and placenta (estrogens and progestins), and testes (testosterone). Steroid hormones are transported by the blood from their sites of synthesis to their target organs. Figure 18.24 Key steroid hormones. 7- Steroid Hormones Because of their hydrophobicity, they must be complexed with a plasma protein. Plasma albumin can act as a nonspecific carrier, and does carry aldosterone. However, specific steroid-carrier plasma proteins bind the steroid hormones more tightly than does albumin, for example, corticosteroid-binding globulin (transcortin) is responsible for transporting cortisol, and sex hormone–binding protein transports sex steroids. A. Synthesis of steroid hormones Synthesis involves shortening the hydrocarbon chain of cholesterol, and hydroxylation of the steroid nucleus. The initial and rate-limiting reaction converts cholesterol to the 21-carbon pregnenolone It is catalyzed by the cholesterol side-chain cleavage enzyme complex (desmolase)—a CYP mixed function oxidase of the inner mitochondrial membrane. NADPH and molecular oxygen are required for the reaction. The cholesterol substrate can be newly synthesized, taken up from lipoproteins, or released from cholesteryl esters stored in the cytosol of steroidogenic tissues. An important control point is the movement of cholesterol into mitochondria. This process is mediated by StAR (steroidogenic acute regulatory protein). A. Synthesis of steroid hormones Note: Steroid hormone synthesis consumes little cholesterol as compared with that required for bile acid synthesis. Pregnenolone is the parent compound for all steroid hormones Pregnenolone is oxidized and then isomerized to progesterone, a progestin, which is further modified to the other steroid hormones by hydroxylation reactions that occur in the ER and mitochondria. Like desmolase, the enzymes are CYP proteins. 17 B. Secretion of steroid hormones from adrenal cortex & gonads Steroid hormones are secreted on demand from adrenal cortex in response to hormonal signals. 1. Cortisol 2. Aldosterone 3. Androgens The testes and ovaries synthesize hormones necessary for physical development and reproduction. The testes to produce testosterone and the ovaries to produce estrogens and progesterone E. Further metabolism of steroid hormones Steroid hormones are generally converted into inactive metabolic excretion products in the liver. Reactions include reduction of unsaturated bonds and the introduction of additional hydroxyl groups. The resulting structures are made more soluble by conjugation with glucuronic acid or sulfate Approximately 20–30% of these metabolites are secreted into the bile and then excreted in the feces, whereas the remainder are released into the blood and filtered from the plasma in the kidney, passing into the urine. These conjugated metabolites are fairly water-soluble and do not need protein carriers. 8- Chapter Summary Cholesterol is a hydrophobic compound, with a single hydroxyl group—located at carbon 3 of the A ring—to which a fatty acid can be attached, producing an even more hydrophobic cholesteryl ester. Cholesterol is synthesized by virtually all human tissues, although primarily by liver, intestine, adrenal cortex, and reproductive tissues (Figure 18.29). All the carbon atoms in cholesterol are provided by acetate, and NADPH provides the reducing equivalents. The pathway is driven by hydrolysis of the high-energy thioester bond of acetyl coenzyme A (CoA) and the terminal phosphate bond of ATP. 8- Chapter Summary Cholesterol is synthesized in the cytoplasm. The rate-limiting and regulated step in cholesterol synthesis is catalyzed by the endoplasmic reticulum–-membrane protein, hydroxymethylglutaryl (HMG) CoA reductase, which produces mevalonic acid from HMG CoA. The enzyme is regulated by a number of mechanisms: 1. Expression of the HMG CoA reductase gene is activated when cholesterol levels are low, resulting in increased enzyme and, therefore, more cholesterol synthesis. 8- Chapter Summary 2. HMG CoA reductase activity is controlled covalently through the actions of an adenosine monophosphate (AMP)–activated protein kinase (AMPK, which phosphorylates and inactivates HMG CoA reductase) and an insulin-activated protein phosphatase (which activates HMG CoA reductase). 3. Statins are competitive inhibitors of HMG CoA reductase. These drugs are used to decrease plasma cholesterol in patients with hypercholesterolemia. The ring structure of cholesterol can not be degraded in humans. 8- Chapter Summary Cholesterol can be eliminated from the body either by conversion to bile salts or by secretion into the bile. Intestinal bacteria can reduce cholesterol to coprostanol and cholestanol, which together with cholesterol make up the bulk of neutral fecal sterols. Bile salts and phosphatidylcholine are quantitatively the most important organic components of bile. Bile salts are conjugated bile acids produced by the liver and stored in the gallbladder. The primary bile acids, cholic or chenodeoxycholic acids, are amphipathic, and can serve as emulsifying agents. 8- Chapter Summary The rate-limiting step in bile acid synthesis is catalyzed by cholesterol-7-α-hydroxylase, which is activated by cholesterol and inhibited by bile acids. Before the bile acids leave the liver, they are conjugated to a molecule of either glycine or taurine, producing the primary bile salts: glycocholic or taurocholic acid, and glycochenodeoxycholic or taurochenodeoxycholic acid. Bile salts are more amphipathic than bile acids and, therefore, are more effective emulsifiers. In the intestine, bacteria can remove the glycine and taurine, and can remove a hydroxyl group from the steroid nucleus, producing the secondary bile acids—deoxycholic and lithocholic acids. 8- Chapter Summary Bile is secreted into the intestine, and more than 95% of the bile acids and salts are efficiently reabsorbed. They are actively transported from the intestinal mucosal cells into the portal blood, where they are carried by albumin back to the liver (enterohepatic circulation). In the liver, the primary and secondary bile acids are reconverted to bile salts, and secreted into the bile. If more cholesterol enters the bile than can be solubilized by the available bile salts and phosphatidylcholine, cholesterol gallstone disease (cholelithiasis) can occur. 8- Chapter Summary The plasma lipoproteins include chylomicrons, very-lowdensity lipoproteins (VLDL), low-density lipoproteins (LDL), and high-density lipoproteins (HDL). They function to keep lipids (primarily triacylglycerol and cholesteryl esters) soluble as they transport them between tissues. Lipoproteins are composed of a neutral lipid core (containing triacylglycerol, cholesteryl esters, or both) surrounded by a shell of amphipathic apolipoproteins, phospholipid, and nonesterified cholesterol. Chylomicrons are assembled in intestinal mucosal cells from dietary lipids (primarily, triacylglycerol) plus additional lipids synthesized in these cells. 8- Chapter Summary Each nascent chylomicron particle has one molecule of apolipoprotein (apo) B-48. They are released from the cells into the lymphatic system and travel to the blood, where they receive apo C-II and apo E from HDLs, thus making the chylomicrons functional. Apo C-II activates lipoprotein lipase, which degrades the chylomicron's triacylglycerol to fatty acids and glycerol. The fatty acids that are released are stored (in the adipose) or used for energy (by the muscle). The glycerol is metabolized by the liver. Patients with a deficiency of lipoprotein lipase or apo C-II show a dramatic accumulation of chylomicrons in the plasma (Type I hyperlipoproteinemia, familial lipoprotein lipase deficiency, or hypertriacylglycerolemia). 8- Chapter Summary After most of the triacylglycerol is removed, apo C-II is returned to the HDL, and the chylomicron remnant—carrying most of the dietary cholesterol—binds to a receptor on the liver that recognizes apo E. The particle is endocytosed and its contents degraded by lysosomal enzymes. Nascent VLDL are produced in the liver, and are composed predominantly of triacylglycerol. They contain a single molecule of apo B-100. Like nascent chylomicrons, VLDL receive apo C-II and apo E from HDL in the plasma. The function of VLDL is to carry triacylglycerol from the liver to the peripheral tissues where lipoprotein lipase degrades the lipid. As triacylglycerol is removed from the VLDL, the particle receives cholesteryl esters from HDL. 8- Chapter Summary This process is accomplished by cholesteryl ester transfer protein. Eventually, VLDL in the plasma is converted to LDL—a much smaller, denser particle. Apo C-II and apo E are returned to HDL, but the LDL retains apo B-100, which is recognized by receptors on peripheral tissues and the liver. LDL undergo receptor-mediated endocytosis, and their contents are degraded in the lysosomes. A deficiency of functional LDL receptors causes Type II hyperlipidemia (familial hypercholesterolemia). The endocytosed cholesterol inhibits HMG CoA reductase and decreases synthesis of LDL receptors. 8- Chapter Summary Some of it can also be esterified by acyl CoA:cholesterol acyltransferase and stored. HDL are created by lipidation of apo A-1 synthesized in the liver and intestine. They have a number of functions, including: 1. serving as a circulating reservoir of apo C-II and apo E for chylomicrons and VLDL; 2. removing unesterified cholesterol from cell surfaces and other lipoproteins and esterifying it using phosphatidylcholine:cholesterol acyl transferase, a liversynthesized plasma enzyme that is activated by apo A-1; and 3. delivering these cholesteryl esters to the liver (“reverse cholesterol transport”). 8- Chapter Summary Cholesterol is the precursor of all classes of steroid hormones (glucocorticoids, mineralocorticoids, and sex hormones— androgens, estrogens, and progestins).