TOXOLOGY OF DRUGS OF ABUSE
advertisement

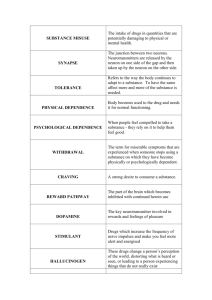
TOXOLOGY OF DRUGS OF ABUSE • The terminology used in discussing drug dependence, abuse, and addiction has long been confusing. • Confusion stems from the fact that repeated use of certain prescribed medications can produce neuroplastic changes resulting in two distinctly abnormal states. • The first is dependence, sometimes called "physical" dependence, produced when there is progressive pharmacological adaptation to the drug resulting in tolerance. 1 TOXOLOGY OF DRUGS OF ABUSE • In the tolerant state, repeating the same dose of drug produces a smaller effect. • If the drug is abruptly stopped: • a withdrawal syndrome ensues in which the adaptive responses are now unopposed by the drug. • Thus, withdrawal symptoms are opposite to the original drug effects. 2 TOXOLOGY OF DRUGS OF ABUSE • The appearance of withdrawal symptoms is the cardinal sign of "physical" dependence. • As thus defined, dependence can occur with the use of: • - opioids • - β blockers • - antidepressants • - benzodiazepines • - stimulants • even when these agents are used as prescribed for therapeutic purposes. 3 TOXOLOGY OF DRUGS OF ABUSE • The state of "physical" dependence is a normal response • easily treatable by tapering the drug dose • and is not in itself a sign of addiction. • The second abnormal state that can be produced by repeated drug use occurs in only a minority of those who initiate drug use. • It leads progressively to compulsive, out-of-control drug use. 4 TOXOLOGY OF DRUGS OF ABUSE • Unfortunately, in 1987 the American Psychiatric Association (APA) chose to use the word "dependence“ • when defining the state of uncontrolled drug use more commonly known as addiction • The word "addiction" was at that time considered pejorative and thus to be avoided. 5 TOXOLOGY OF DRUGS OF ABUSE • The result, over the last two decades, is that confusion has developed between dependence as a normal response and dependence as addiction. • The newest version of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) due to be released in 2012 will correct this confusion. 6 TOXOLOGY OF DRUGS OF ABUSE • This distinction between dependence and addiction is important • because patients with pain sometimes are deprived of adequate opioid medication • simply because they have shown evidence of tolerance • or they exhibit withdrawal symptoms if the analgesic medication is stopped or reduced abruptly. 7 TOXOLOGY OF DRUGS OF ABUSE • Modern neuroscience has greatly increased our understanding of the phenomenology of addiction. • Using animal models as well as human brain imaging studies and clinical observations, addiction can be defined fundamentally as a form of maladaptive memory. • It begins with the administration of substances (e.g., cocaine) or behaviors (e.g., the thrill of gambling) that directly and intensely activate brain reward circuits. • Activation of these circuits motivates normal behavior and most humans simply enjoy the experience without 8 being compelled to repeat it. TOXOLOGY OF DRUGS OF ABUSE 9 ADDICTION • 1- Addiction involves progressive loss of frontal cortical • 2- behavioral control and increasing limbic temporal lobe • 3- negative feelings • The loss of behavioral control that underlies addiction has at least two components 10 ADDICTION • decreased frontal cortical regulation of attention and cognitive flexibility and increased limbic fearnegative feelings (Fig. 1). • The frontal lobes of brain are involved in decision • making and other executive functions such as motivation 11 TOXOLOGY OF DRUGS OF ABUSE • CNS depressants, including: • • • • • • - alcohol - other sedatives hyponotics such as Barbiturates - nicotine and tobacco - opioids; cannabinoids - psychedelic drugs; - inhalants (volatile solvents, nitrous oxide, and ethyl ether) 12 TOXOLOGY OF DRUGS OF ABUSE • CNS Depressants • Ethanol - Experimentation with is almost universal, and a high proportion of users find the experience pleasant. • - Ethanol is classified as a depressant because it indeed • produces sedation and sleep. - However, the initial effects of alcohol, particularly at • lower doses, often are perceived as stimulation owing to a suppression of inhibitory systems in the brain. 13 TOXOLOGY OF DRUGS OF ABUSE • CNS Depressants • Those who perceive only sedation from alcohol generally choose not to drink when evaluated in a test procedure. • Alcohol impairs recent memory • in high doses, produces the phenomenon of "blackouts": • the drinker has no memory of his or her behavior while intoxicated. 14 TOXOLOGY OF DRUGS OF ABUSE • CNS Depressants • The effects of alcohol on memory are unclear • but evidence suggests that reports from patients about their reasons for drinking and their behavior during a binge are not reliable. • Alcohol-dependent persons often say that they drink to relieve anxiety or depression. • When allowed to drink under observation, however, alcoholics typically become more dysphoric as drinking continues • thus not supporting the idea that alcoholics drink to relieve 15 tension. TOXOLOGY OF DRUGS OF ABUSE • CNS Depressants • Tolerance, Physical Dependence, and Withdrawal • Mild intoxication by alcohol is familiar to almost everyone, but the symptoms vary among individuals. • • Some simply experience motor incoordination and sleepiness. • Others initially become stimulated and garrulous. • As the blood level increases, the sedating effects increase, with eventual coma and death occurring at 16 high alcohol levels. TOXOLOGY OF DRUGS OF ABUSE • CNS Depressants • Tolerance, Physical Dependence, and Withdrawal • The initial sensitivity (innate tolerance) to alcohol varies greatly among individuals and is related to family history of alcoholism • Experience with alcohol can produce greater tolerance (acquired tolerance) such that extremely high blood levels (300-400 mg/dL) can be found in alcoholics who do not appear grossly sedated. • In these cases, the lethal dose does not increase proportionately to the sedating dose • thus the margin of safety (therapeutic index) is decreased. 17 TOXOLOGY OF DRUGS OF ABUSE • CNS Depressants • Tolerance, Physical Dependence, and Withdrawal • Heavy consumers of alcohol not only acquire tolerance but also inevitably develop a state of physical dependence. • • This often leads to drinking in the morning to restore blood alcohol levels diminished during the night. • Eventually, they may awaken during the night and take a drink to avoid the restlessness produced by falling alcohol levels. 18 TOXOLOGY OF DRUGS OF ABUSE • CNS Depressants • Tolerance, Physical Dependence, and Withdrawal • The alcohol-withdrawal syndrome generally depends on the size of the average daily dose • and usually is "treated" by resumption of alcohol ingestion. • Withdrawal symptoms are experienced frequently but usually are not severe or life-threatening until they occur in conjunction with other problems • such as infection, trauma, malnutrition, or electrolyte imbalance. • In the setting of such complications, the syndrome of delirium tremens becomes likely. 19 Signs and Symptoms of CNS Depressant Intoxication and Withdrawal • Intoxication (similar to alcohol) • Disinhibition (e.g., inappropriate sexual or aggressive behavior, impaired judgment, mood lability) • Somnolence, stupor, or coma • Impaired attention or memory • Slurred speech • Incoordination • Unsteady gait 20 • Nystagmus Signs and Symptoms of CNS Depressant Intoxication and Withdrawal • Alcohol or any CNS DEPRESSANTS Withdrawal Syndrome • Alcohol craving • Tremor, irritability • Nausea • Sleep disturbance • Tachycardia • Hypertension • Perceptual distortion 21 • Continue …….Alcohol or any CNS DEPRESSANTS Withdrawal Syndrome • Seizures (6-48 hours after last drink) • Visual (and occasionally auditory or tactile) hallucinations (12-48 hours after last drink) • Delirium tremens (48-96 hours after last drink; rare in uncomplicated withdrawal) • Severe agitation • Confusion • Fever, profuse sweating • Diarrhea • Dilated pupils 22 • Continue …….Alcohol or any CNS DEPRESSANTS Withdrawal Syndrome • Delirium tremens Abbreviation: DT - The most severe expression of alcohol withdrawal syndrome marked by: • - visual, auditory, or tactile hallucinations • - extreme disorientation • - restlessness, and hyperactivity of the autonomic nervous system (evidenced by such findings as pupillary dilation - fever, tachycardia, hypertension, and profuse sweating). • - About 15% of affected patients may die, usually as a result of comorbid illnesses. In most affected patients, recovery occurs within 3 to 5 days. SYN:alcoholicdelirium 23 • • 24 • Alcohol addiction produces cross-tolerance to other sedatives such as benzodiazepines. • This tolerance is operative in abstinent alcoholics, • but while the alcoholic is drinking, the sedating effects of alcohol add to those of other sedatives, making the combination more dangerous. • This is particularly true for benzodiazepines, which are relatively safe in overdose when given alone but potentially are lethal in combination with alcohol. 25 • The chronic use of alcohol and other sedatives is associated with the development of depression • The risk of suicide among alcoholics is one of the highest of any diagnostic category. • Cognitive deficits have been reported in alcoholics tested while sober. • These deficits usually improve after weeks to months of abstinence. • More severe recent memory impairment is associated with specific brain damage caused by nutritional deficiencies common in26 alcoholics due to thiamine deficiency). • Alcohol is toxic to many organ systems. • As a result, the medical complications of alcohol abuse and dependence include liver disease • cardiovascular disease, • endocrine and GI effects, and malnutrition • Alcohol crosses the placental barrier, producing the fetal alcohol syndrome, a major cause of mental retardation to the fetus 27 • Pharmacological Interventions • Detoxification • A patient who presents in a medical setting with an alcohol-withdrawal syndrome should be considered to have a potentially lethal condition. • Although most mild cases of alcohol withdrawal never come to medical attention • severe cases require general evaluation; attention to hydration and electrolytes; vitamins, • especially high-dose tthiamine; and a sedating medication that has cross-tolerance with alcohol. 28 • Pharmacological Interventions • Detoxification • To block or diminish the symptoms of alcohol toxicity, a shortacting benzodiazepine such as oxazepam can be used at a dose of 15-30 mg every 6-8 hours according to the stage and severity of withdrawal • A patient who presents in a medical setting with an alcoholwithdrawal syndrome should be considered to have a potentially lethal condition. • some authorities recommend a long-acting benzodiazepine unless there is demonstrated liver impairment. • Anticonvulsants such as carbamazepine have been shown to be 29 effective in alcohol withdrawal • Pharmacological Interventions • Pharmacotherapy • Detoxification is only the first step of treatment. • Complete abstinence is the objective of long-term treatment, and this is accomplished mainly by behavioral approaches. • Medications that aid in the prevention of relapse are under development. • Disulfiram (ANTABUSE; has been useful in some programs that focus behavioral efforts on ingestion of the medication 30 • Pharmacological Interventions • Pharmacotherapy • Disulfiram blocks aldehyde dehydrogenase, the second step in ethanol metabolism, resulting in the accumulation of acetaldehyde • which produces an unpleasant flushing reaction when alcohol is ingested. • Knowledge of this unpleasant reaction helps the patient to resist taking a drink. • Although quite effective pharmacologically, disulfiram has not been found to be effective in controlled clinical trials because so many patients failed to ingest the medication. 31 • Pharmacological Interventions • Naltrexone (REVIA) an opioid receptor antagonist that blocks the reinforcing properties of alcohol, is FDA- approved as an adjunct in the treatment of alcoholism. • • Chronic administration of naltrexone resulted in a decreased rate of relapse to alcohol drinking in the majority of published doubleblind clinical trials . It works best in combination with behavioral treatment programs that encourage adherence to medication and abstinence from alcohol. • A depot preparation with a duration of 30 days (VIVITROL) was approved by the FDA in 2006; it greatly improves medication adherence, the major problem with the use of medications in alcoholism. 32 • Pharmacological Interventions • A significant development in identifying a potential endophenotype of alcoholism has grown out of the clinical experience with naltrexone. • Animal studies have demonstrated that alcohol causes the release of endogenous opioids in brain reward systems • disinhibition or activation of dopamine neurons, a condition common to all drugs of abuse. • Blocking opioid receptors prevents this dopaminergic effect and results in less stimulation or reward from alcohol. 33 • Pharmacological Interventions • A functional allele of the gene for the μ opioid receptor that naltrexone blocks has been associated with alcohol stimulation and with good response to naltrexone treatment among alcoholics. • Acamprosate (campral), another FDA-approved medication for alcoholism , is a competitive inhibitor of the N-methyl-D-aspartate (NMDA)–type glutamate receptor. • The drug appears to normalize the dysregulated neurotransmission associated with chronic ethanole intake and thereby to attenuate one of the mechanisms that lead to relapse 34 • Pharmacological Interventions • Barbiturates and Older Sedatives The use of barbiturates and older non-benzodiazepine sedating medications such as: • - chloral-hydrate • - meprobamatee • - glutethimide • • has declined greatly in recent years owing to the increased safety and to the efficacy of the benzodiazepines and the newer agents ZOLPIDEM , ESZOPICLONE , AND ZALEPLON • Abuse problems with barbiturates resemble those seen with benzodiazepines in many ways 35 • Pharmacological Interventions . Treatment of abuse and addiction should be handled similarly to interventions for the abuse of alcohol and benzodiazepines. • Because drugs in this category frequently are prescribed as hypnotics for patients complaining of insomnia, physicians should be aware of the problems that can develop when the hypnotic agent is withdrawn. • Insomnia rarely should be treated with medication as a primary disorder except when produced by short-term stressful situations. 36 • Pharmacological Interventions . . Insomnia often is a symptom of an underlying chronic problem, such as: - depression or respiratory dysfunction, or may be due simply to a change in sleep requirements with age. - Prescription of sedative medications, however, can change the physiology of sleep with subsequent tolerance to these medication effects. - When the sedative is stopped, there is a rebound effect with worsened insomnia. This medication-induced insomnia requires detoxification by gradual dose . 37 Pharmacological Treatment of Withdrawal Syndromes from Substances of Abuse Substance Alcohol Other CNS depressants 38 Agent and Dosage Other Treatment Thiamine, 100 mg Diazepam, 10–20 intramuscularly or mg/1–2 hours 50 mg twice daily by (typical dosage mouth, and required, 60 mg) multivitamin tablets for 3 days Phenobarbital, 120 mg/hour (typical dosage, 900–1500 mg) Opiates andOpioids 39 Opioid receptor subtypes, their functions 1- μ (mu) • Supraspinal and spinal analgesia; • sedation; inhibition of respiration • slowed gastrointestinal transit • modulation of hormone and neurotransmitter release 2- δ (delta) • Supraspinal and spinal analgesia; • modulation of hormone • and neurotransmitter release 40 Opioid receptor subtypes, their functions 3- κ (kappa) • Supraspinal and spinal analgesia • Psychotomimetic effects • slowed gastrointestinal transit 41 Opioids • Opioid drugs are used primarily for the treatment of pain • Some of the CNS mechanisms that reduce the perception of pain also produce a state of well-being or euphoria. • Thus, opioid drugs also are taken outside medical channels for the purpose of obtaining the effects on mood. • This potential for abuse has generated much research on separating the mechanism of analgesia from that of euphoria • in the hope of eventually developing a potent analgesic that does not activate the brain reward systems. 42 Opioids • options for the treatment of pain, but none of these currently is available for clinical use. • Progress in pain control stems from a greater understanding of the mechanism of tolerance to μ opiate receptor–mediated analgesia, which involves NMDA receptors • Experimentally, by combining morphine with dextromethorphan • , an NMDA- receptor antagonist, tolerance is impaired and analgesia is enhanced without an increase in the dose of opioid. 43 • Opioids Tolerance, Dependence, and Withdrawal • Injection of a heroin solution produces a variety of sensations described as warmth, taste, or high and intense pleasure ("rush") often compared with sexual orgasm. • There are some differences among the opioids in their acute effects, with morphine producing more of a histamine-releasing effect • and meperidine producing more excitation or confusion. 44 • Opioids Tolerance, Dependence, and Withdrawal • Heroin has high lipid solubility, crosses the blood-brain barrier quickly • and is deacetylated to the active metabolites 6monoacetyl morphine and morphine. • After the intense euphoria, which lasts from 45 seconds to several minutes, • there is a period of sedation and tranquility ("on the 45 nod") lasting up to an hour. • Opioids Tolerance, Dependence, and Withdrawal • The effects of heroin wear off in 3-5 hours, depending on the dose. • Experienced users may inject two to four times per day. • Thus, the heroin addict is constantly oscillating between being "high" and feeling the sickness of early withdrawal • This produces many problems in the homeostatic 46 systems regulated at least in part by endogenous opioids. • For example, the hypothalamic-pituitary-gonadal axis and the hypothalamic-pituitary-adrenal axis are abnormal in heroin addicts. • Women on heroin have irregular menses, • and men have a variety of sexual performance problems. • Mood also is affected. Heroin addicts are relatively docile and compliant after taking heroin, • but during withdrawal, they become irritable and aggressive. 47 • Based on patient reports, tolerance develops early to the euphoria-producing effects of opioids. • There also is tolerance to the respiratory depressant, analgesic, sedative, and emetic properties. • Heroin users tend to increase their daily dose, depending on their financial resources and the availability of the drug. • If a supply is available, the dose can be increased progressively 100 times. 48 OPIOIDS • Heroin users commonly acquire: • bacterial infections producing skin abscesses; • endocarditis; • pulmonary infections, especially tuberculosis; and viral infections producing hepatitis C and acquired immune deficiency syndrome (AIDS). 49 OPIOIDS The opioid-withdrawal syndrome is very unpleasant but not life-threatening. It begins within 6-12 hours after the last dose of a short-acting opioid and as long as 72-84 hours after a very long-acting opioid medication. Heroin addicts go through early stages of this syndrome frequently when heroin is scarce or expensive 50 Signs and Symptoms of Opioid Intoxication and Withdrawal Intoxication Withdrawal Activation or “rush” (early or with low dosages) and sedation/apathy or Depressed mood and anxiety “nod” (late or with high dosages) Dysphoria Euphoria or dysphoria Craving Feelings of warmth, facial flushing, or Piloerection (“goose flesh”) itching Lacrimation or rhinorrhea Impaired judgment, attention, or 51 memory OpioidIntoxication Opioid-Withdrawal Analgesia Hyperalgia, joint and muscle aches Constipation Diarrhea and gastrointestinal cramping, nausea, or vomiting Pupillary constriction Pupillary dilation and photophobia Drowsiness Insomnia Respiratory depression, areflexia, hypotension, tachycardia Autonomic hyperactivity (e.g., tachypnea, hyperreflexia, tachycardia, hypertension, sweating, hyperthermia) Apnea, cyanosis, coma 52 Yawning Pharmacological Treatment of Withdrawal Syndromes from Substances of Abuse Substance Agent and Dosage Other Treatment 3–5 days of clonidine 0.1–0.3 mg every 4–6 hours (check BP prior to each dose, hold for BP ≤90/60) Opioids 53 Alternatively, methadone dosed at 10–20 mg Ibuprofen for muscle by mouth every 12 hours initially, or cramps, loperamide buprenorphine dosed at 4–12 mg under for loose stools, and tongue daily initially, both taper over a 5– promethazine for 10-day period to reduce withdrawal nausea or vomiting symptoms (1 mg buprenorphine is equivalent to 5 mg methadone, 5 mg of heroin, 15 mg of morphine, 100 mg of meperidine) Pharmacological Maintenance Strategies for Substance afterand Detoxification SubstanceDependenceAgent Dosage Completed Methadone by mouth at 30–140 mg per day Opioids 54 Buprenorphine 4–32 mg under the tongue (available as buprenorphine/naloxone [4/1] to prevent diversion) Cannabinoids • Marijuana is the common name for the plant Cannabis sativa. • Other names for the plant or its products include hemp, hashish, chasra, bhang, ganja, and daga. • The highest concentrations of the psychoactive cannabinoids are found in the flowering tops of both male and female plants. • The primary psychoactive constituent of marijuana is delta-9-tetrahydrocannabinol 55 Cannabinoids • Cannabinoid receptors CB1 (mainly CNS) and CB2 (peripheral) have been identified and cloned. • An arachidonic acid derivative, anandamide, has been proposed as an endogenous ligand for CB receptors. • While the physiological function of these receptors and their endogenous ligands are incompletely understood • they are likely to have important functions because they are dispersed widely with high densities in the cerebral cortex, hippocampus, striatum, and cerebellum . 56 Cannabinoids • Specific CB1 antagonists have been developed and tested in controlled clinical trials. • One of these, rimonabant, was found to reduce: • 1 - relapse in cigarette smokers • 2- to produce weight loss in obese patients • however, its development has been abandoned because of depressive and neurologic side effects. 57 Cannabinoids • Intoxication • The subjective effect of marijuana intoxication varies from individual to individual. • It is determined in part by highly variable pharmacokinetics, dosage, route of administration, setting, experience and expectation, • and individual vulnerability to certain psychotoxic effects. • Typically, intoxication is characterized by an initial period of “high” that has been described as a sense of well-being and happiness . 58 Cannabinoids • Intoxication • This euphoria is followed frequently by a period of drowsiness or sedation. • The perception of time is altered and hearing and vision distorted. • The subjective effects of intoxication often include dissociative reactions. • Impaired functioning occurs in a variety of cognitive and performance tasks, • including memory, reaction time, concept formation, learning, perception, motor coordination, attention, and signal detection. 59 Cannabinoids • Intoxication At dosages equivalent to one or two “joints” (marijuana cigarettes), processes involved in the operation of motor vehicles or airplanes are impaired. The impairment persists for 4–8 hours, long after the user perceives the subjective effects of the drug. The impairment produced by alcohol is additive to that produced by marijuana. Tolerant individuals may exhibit somewhat less performance decrement. 60 Cannabinoids • . Signs and Symptoms of Cannabis Intoxication • Euphoria, drowsiness, or sedation • Sensation of slowed time • Auditory or visual distortions, dissociation • Impaired judgment, motor coordination, attention, or memory • Slowed reaction time • Conjunctival injection (dilation of blood vessel of conjuctiva) • Tachycardia • Increased appetite • Anxiety, acute panic reactions, paranoia, illusions, or agitation 61 Cannabinoids • . Signs and Symptoms of Cannabis Intoxication • Physically, dilation of conjunctival blood vessels and tachycardia may be noted. • Blood pressure remains relatively unchanged unless high dosages are used, in which case orthostatic hypotension ensues. • Increased appetite is often attributed to marijuana but has not been observed consistently in controlled studies. 62 Cannabinoids • . Signs and Symptoms of Cannabis Intoxication • At higher dosages, • acute panic reactions, paranoia, • hallucinations, illusions, thought disorganization, and agitation have been observed. • With extremely high dosages, an acute toxic psychosis is accompanied by: • depersonalization and loss of insight. 63 Cannabinoids • One of the most controversial of the reputed effects of marijuana is the production of • an "amotivational syndrome.“ • It has anti-emetic properties that relieve side effects of anticancer chemotherapy. • It also has muscle-relaxing effects, anticonvulsant properties, • and the capacity to reduce the elevated intraocular pressure of glaucoma. 64 Cannabinoids • These medical benefits come at the cost of the psychoactive effects that often impair normal activities. • Thus, there is no clear advantage of marijuana over conventional treatments for any of these indications. • An oral capsule containing Δ-9-THC (dronabinol; MARINOL, others) is approved for : • - anorexia associated with weight loss in patients with HIV infection and • - for cancer chemotherapy-induced nausea and vomiting. 65 Cannabinoids • With the cloning of cannabinoid receptors, the discovery of endogenous ligands, and the synthesis of specific agonists and antagonists, • it is likely that new orally effective medications will be developed without the undesirable properties of smoked marijuana and • without the deleterious effects of inhaling smoke particles and the chemical products of high-temperature combustion. 66 Cannabinoids • . Signs and Symptoms of Cannabis Withdrawal • Cannabinoid withdrawal does not produce wellcharacterized withdrawal symptoms, • perhaps because cannabinoids are so lipophilic that they are very slowly eliminated from the body. • The DSM-IV does not include cannabis withdrawal, but there is an impetus to include the condition in future versions of DSM. 67 Cannabinoids • . Signs and Symptoms of Cannabis Withdrawal • a withdrawal syndrome consistently follows discontinuation of chronic heavy use of cannabis, • or treatment with cannabinoid receptor antagonists. • Some patients report: • insomnia, irritability, dysphoria, anorexia, weight loss, hand tremor, mild fever, or slight nausea with discontinuation of use. • These symptoms occur primarily in patients who smoke 68 very potent preparations. Pharmacological Treatment of Withdrawal Syndromes from Substances of Abuse Substance Cannabinoids 69 Agent and Dosage Not usually needed Other Treatment Anxiolytics or neuroleptics acutely for agitation or severe anxiety Animal Models of Substance Abuse and Addiction • The reinforcing effects of drugs of abuse are believed to play • a key role in substance abuse and addiction. • Early demonstrations that drugs could serve as reinforcers maintaining operant behavior in laboratory animals led to the development of a model of human drug abuse. • the paradigms used for establishing drugs as reinforcers in • Animals focusing on: 1- drug self-administration 2- conditioned place preference 3- drug discrimination paradigms. 70 Animal Models of Substance Abuse and Addiction • focused on modeling different phases of the addiction process • In Animal models often focus on the ability of the drugs to directly control the animal’s behavior • an outcome that is consistent with the behavioral definition of addiction. • animal studies have demonstrated that the rewarding effect is not dependent on preexisting conditions; that is, exposure to the drug is sufficient to motivate drug-taking behavior. • Self-administration by laboratory animals of drugs abused by humans also supports the concept that drugs act as universal reinforcers. 71 • Drug self-administration paradigm. • The traditional animal models of drug abuse are framed by the behaviorist view that emphasizes • the action of drugs as positive reinforcers, much like food, • water, and other ‘natural’ reinforcers. • The fundamental principle is that aspects of behavior are controlled by their consequences. • A drug is said to be functioning as a reinforcer if responding • for it is maintained above responding for saline or other control • conditions. 72 • Drug self-administration paradigm. • • The traditional model entails training an animal to • self-administer a drug during a short daily session, typically 1 • to 3 h. • Figure shows a rat in a typical operant chamber with an • intravenous catheter for chronic selfadministration. • 73 74 • Drug self-administration paradigm. • Rodents are most often used in these studies • this model has been used with a variety of species including nonhuman primates, • dogs, and cats. • In rodents, a low-ratio requirement typically is used, such as a fixed-ratio 1 • where each operant response produces a drug delivery. • In addition, a variety of operant responses have been used. • Typically they depend on the species studied (for • example, a lever press or a nose poke response typically is used for rodents, • whereas a panel press response typically is used for nonhuman primates). 75 • The most common routes of administration are intravenous • and oral, but intracerebroventricular, intracranial, inhalation, intragastric,and intramuscular routes have also been used • some of these other routes (for example, smoked) are used relatively infrequently • because of practical and logistical difficulties. • • Generally, these studies use the route of administration that is most similar to the route used in humans for that particular drug. For example, animal studies with alcohol typically use an oral route of administration, • whereas an intravenous route is used for drugs like cocaine, • heroin, and nicotine, to mimic the rapid onset produced 76 • by intravenous or inhalational administration in • • Taste factors must often be considered with the oral route, given that these often limit consumption of pharmacologically active doses • however, use of intragastric self-administration or sweetening an • alcohol solution with saccharin are 2 methods used to avoid the • influence of taste. • Results from animal drug self-administration studies have revealed that drugs can serve as positive reinforcers • and there appears to be good correspondence between humans • and animals in terms of drugs that are self-administered • and patterns of drug intake. 77 • For example, drugs that are abused by humans generally maintain responding in animals • whereas drugs that do not maintain responding in animals typically are not abused by humans • In addition, similar patterns of drug intake have been reported in humans and animals for ethanol, opioids, nicotine, and cocaine self-administration. • These parallel results between the human and animal drug literature validate the animal model of drug abuse • and suggest that the use of this model may lead to a better understanding of human drug-taking behavior. 78 • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • The traditional self-administration procedure has been instrumental in characterizing the brain regions and signaling pathways that are responsible for rewarding behaviors. This ‘reward pathway’ is comprised of several brain regions, the most prominent being the ventral tegmental area, nucleus accumbens, and Vol 60, No 3 Comparative Medicine June 2010 180 ence 83). Early studies with monkeys and rats showed that, like those in humans, patterns of self-administration in laboratory animals that are given unlimited access conditions (that is, 24-h sessions wherein each response was reinforced under a fixedratio 1 schedule) were characterized by dysregulated and binge patterns of use. Under these conditions, toxicity can develop rapidly, particularly with unlimited access to psychostimulant drugs and opiates, thereby necessitating the use of procedures that limit access to these drugs in some way. Recent studies have attempted to capture these features—dysregulated patterns of use and excessive consumption—without the toxicity. For example, dysregulated and excessive drug intake without serious toxicity has been observed to occur under 24-h access conditions with low-unit doses of drug18 and under continuous access conditions that limit the number of hours of access each day (that is, 6 to 12 h daily1) or each period of continuous access (that is, 72 h).101 Another method that has been used with limited toxicity is to give animals 24-h access to a drug in discrete trials throughout the light:dark cycle.27 This method has been used for cocaine self-administration, and the results have shown that 79the regularity of patterns of use break down and intake progressively increases as access conditions increase. Under shortaccess • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • Drug self-administration paradigm. The traditional animal models of drug abuse are framed by the behaviorist view that emphasizes the action of drugs as positive reinforcers, much like food, water, and other ‘natural’ reinforcers. The fundamental principle is that aspects of behavior are controlled by their consequences. A drug is said to be functioning as a reinforcer if responding for it is maintained above responding for saline or other control conditions. The traditional model entails training an animal to self-administer a drug during a short daily session, typically 1 to 3 h. Figure 2 shows a rat in a typical operant chamber with an intravenous catheter for chronic self-administration. Although rodents are most often used in these studies, this model has been used with a variety of species including nonhuman primates, dogs, and cats. In rodents, a low-ratio requirement typically is used, such as a fixed-ratio 1, where each operant response produces a drug delivery. In addition, a variety of operant responses have been used. Typically they depend on the species studied (for example, a lever press or a nose poke response typically is used for rodents, whereas a panel press response typically is used for nonhuman primates). The most common routes of administration are intravenous and oral, but intracerebroventricular, intracranial, inhalation, intragastric, and intramuscular routes have also been used; some of these other routes (for example, smoked) are used relatively infrequently, because of practical and logistical difficulties. Generally, these studies use the route of administration that is most similar to the route used in humans for that particular drug. For example, animal studies with alcohol typically use an oral route of administration, whereas an intravenous route is used for drugs like cocaine, heroin, 80 and nicotine, to mimic the rapid onset produced by intravenous or inhalational administration in humans.19 Taste • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • Drug self-administration paradigm. The traditional animal models of drug abuse are framed by the behaviorist view that emphasizes the action of drugs as positive reinforcers, much like food, water, and other ‘natural’ reinforcers. The fundamental principle is that aspects of behavior are controlled by their consequences. A drug is said to be functioning as a reinforcer if responding for it is maintained above responding for saline or other control conditions. The traditional model entails training an animal to self-administer a drug during a short daily session, typically 1 to 3 h. Figure 2 shows a rat in a typical operant chamber with an intravenous catheter for chronic self-administration. Although rodents are most often used in these studies, this model has been used with a variety of species including nonhuman primates, dogs, and cats. In rodents, a low-ratio requirement typically is used, such as a fixed-ratio 1, where each operant response produces a drug delivery. In addition, a variety of operant responses have been used. Typically they depend on the species studied (for example, a lever press or a nose poke response typically is used for rodents, whereas a panel press response typically is used for nonhuman primates). The most common routes of administration are intravenous and oral, but intracerebroventricular, intracranial, inhalation, intragastric, and intramuscular routes have also been used; some of these other routes (for example, smoked) are used relatively infrequently, because of practical and logistical difficulties. Generally, these studies use the route of administration that is most similar to the route used in humans for that particular drug. For example, animal studies with alcohol typically use an oral route of administration, whereas an intravenous route is used for drugs like cocaine, heroin, 81 and nicotine, to mimic the rapid onset produced by intravenous or inhalational administration in humans.19 Taste • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • in characterizing the brain regions and signaling pathways that are responsible for rewarding behaviors. This ‘reward pathway’ is comprised of several brain regions, the most prominent being the ventral tegmental area, nucleus accumbens, and Vol 60, No 3 Comparative Medicine June 2010 180 ence 83). Early studies with monkeys and rats showed that, like those in humans, patterns of self-administration in laboratory animals that are given unlimited access conditions (that is, 24-h sessions wherein each response was reinforced under a fixedratio 1 schedule) were characterized by dysregulated and binge patterns of use. Under these conditions, toxicity can develop rapidly, particularly with unlimited access to psychostimulant drugs and opiates, thereby necessitating the use of procedures that limit access to these drugs in some way. Recent studies have attempted to capture these features—dysregulated patterns of use and excessive consumption—without the toxicity. For example, dysregulated and excessive drug intake without serious toxicity has been observed to occur under 24-h access conditions with low-unit doses of drug18 and under continuous access conditions that limit the number of hours of access each day (that is, 6 to 12 h daily1) or each period of continuous access (that is, 72 h).101 Another method that has been used with limited toxicity is to give animals 24-h access to a drug in discrete trials throughout the light:dark cycle.27 This method has been used for cocaine self-administration, and the results have shown that the regularity of patterns of use break down and intake progressively increases as access conditions increase. Under shortaccess 82 conditions (that is, 1 to 2 discrete trials per hour, 1.5 mg/