MATTER Unit - Solon City Schools

Bellringers
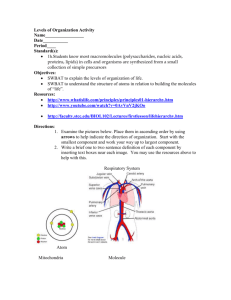
Intro to
MATTER
UNIT
MATTER Unit
Monday, September 26, 2011
• Objective: SWBAT compare and contrast pure substances and mixtures.
• BR: What is an atom? Do you know what is formed when atoms combine?
• Lesson:
1. Discuss reading about atoms from Friday
2. Notes on Pure Substances vs Mixtures
3. Read pp 38-44
4. Start HW (questions 1-7 on p 44)
• HW: questions 1-7 on p 44
MATTER Unit
Tuesday, September 27
• Objective: SWBAT compare and contrast elements and compounds and heterogeneous and homogeneous mixtures.
• BR: Label the following as pure substances or mixtures:
1. Elements
2. Colloid
3. Atoms
4. Suspension
5. Compounds
6. Sand on a beach
7. Solutions
8. Milk
9. Copper
• Lesson:
1.
HW Quiz
2.
Finish notes (mixtures)
• HW: Section Review 2.1 (front and back)
MATTER Unit
Wednesday
September 28
• Objective: SWBAT review types of matter and how to calculate density.
• BR: Make a venn diagram to compare and contrast homogeneous and heterogeneous mixtures. Be sure to write in examples.
• Lesson:
1. Go over BR
2. Stations Lab
3. Go over Lab, HW questions from last night?
4. Practice Density Problems (p 48: 1-3)
5. Go over density problems
• HW: DENSITY PROBLEMS P. 61 # 23-26
• NO SCHOOL THURSDAY
MATTER Unit
Friday, September 30
• Objective: SWBAT compare and contrast physical and chemical properties and changes.
• BR: 1. What is a physical property; a chemical property?
• Lesson:
1. Discovery Ed Video Lesson on Physical vs Chemical
Properties
2. Notes on Chemical/Physical Properties
• HW:
– DENSITY PROBLEMS P. 61 # 23-26 (From Wed-quiz Mon)
– page 58 # 1, 2, 7
MATTER Unit
Monday, October 3
• Objective: SWBAT measure and calculate the density of a liquid, regular solid and irregular solid using proper SI units.
• BR: 1. What is density. 2. Is density a chemical or physical property? 3. What units do we use to report density?
• LESSON
1.
Density Quiz
2.
Go over BR and how to use the water displacement method
3.
Density of a solid/liquid Lab
1.
Watch your precision and sig figs in all calculations!!!
2.
Remember – when do you need to use water displacement to find volume? When do you NOT?
• HW: finish lab (there is a back)
MATTER Unit
Tuesday
, October 4
• Objective: SWBAT create a mass-volume graph for values collected in the lab.
• BR: What is the formula for calculating density? What are the two units we can use for density?
• Lesson:
1. Discuss density lab
2. Penny Lab
• HW: Finish the graph
MATTER Unit
Wednesday
, October 5
• Objective: SWBAT interpolate and extrapolate data about the density of pennies pre- and post -1982.
• BR: Draw the set up for a graph with mass on the y axis and volume on the x axis? What is the slope of this line?
• Lesson:
1. Make Graph(s)
2. Finish Penny Lab
• HW: Finish Lab
MATTER Unit
Thursday, October 6
• Objective: SWBAT identify and list examples of chemical and physical properties and changes.
• BR: What is the difference between a physical change and a physical property. Give an example of each.
• Lesson:
1. Notes on Physical/Chemical Properties and changes (2 nd period- present posters)
• HW: Section review 2.2
MATTER Unit
Friday
, October 7
• Objective: SWBAT explain how density of a liquid relates to its viscosity.
• BR:
1. Define: density and viscosity
2. Label the following as a Chemical/Physical Property or Change.
A.
Dissolving sugar in water
B.
Adding Acid to a base to make water
C.
Burning a piece of wood
D.
Flammability
• Lesson:
E. Solubility
F. Color
G. Baking cookies
H. Density
1.
Demo Density Tower
2.
Chemical Change Demo
3.
Viscosity and Density Lab
• HW: Study Guide
1. The following liquids are placed into a density tower. Draw it.
2. Pretend I drop in a ball with a density of .93. Where would you draw it?
Material
• Light Corn Syrup (Brown)
• Rubbing Alcohol (clear)
• Water with food color(clear)
• Vegetable Oil (yellow)
• Dawn Dish Soap (purple)
Density (g/mL)
1.33 g/mL
.79 g/mL
1.00 g/mL
.92 g/mL
1.06 g/mL
MATTER Unit
Monday, October 10
• Y O U D O N O T N E E D T O W R I T E A N Y T H I N G O N Y O U R B R
S H E E T - J U S T S TA P L E I T T O Y O U R B R ’ S o n T E S T D AY ! ! !
• Objective: REVIEW
• BR: Pick up and complete “Mass vs. Volume of Aluminum.” (pink)
• Lesson:
1.
Go over BR
2.
Clicker
3.
Work in groups
• Make a concept map from memory of the following words: Matter, Pure
Substance, Mixture, Element, Compound, Homogeneous Mixture,
Solution, Heterogeneous Mixture, Colloid, Suspension, Tyndall Effect
• Complete worksheets to help review
• HW: STUDY!!!
Thursday October 13th
• TODAY IS OUR TEST!!!
• Please clear your desks of EVERYTHING but a writing utensil and get a divider for your desk.
• Get ready to hand in…
– 1. Bellringers
– 2.)