Monday, November 29 - Chemistry at Winthrop University
advertisement

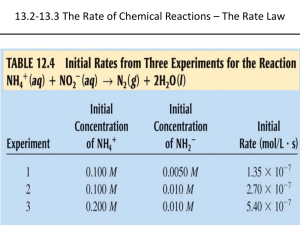
Chemical Kinetics • Kinetics: The Study of the rate of chemical reactions • Thermodynamics: The study of the energy associated with chemical reactions • Remember: Thermodynamics is concerned with state functions (where we started and where we finished) but Kinetics is concerned with how we get to where we finish. The Rate of a Chemical Reaction What is a rate? A rate is a change in a property per unit time. For example, how do we define the speed of a ski boat? There are 2 types of speed: 1. Average speed: The total distance traveled divided by the total time 2. Instantaneous speed: Looking at the speedometer on the boat tells you the rate you are traveling RIGHT THEN The Reaction Rate The reaction rate is the change in the rate of formation of products OR consumption of reactants per unit time [R] 2 [R]1 -[Reactants] Rate = = - Note : The minus sign t t 2 t1 [Products] [P]2 [P]1 Rate = = t t Reaction Rate and Stoichiometry • In the example: 2NH3 (g) --> N2 (g) + 3H2 (g) The rate of formation of N2 is one half the rate of disappearance of NH3. Prove this. Use the previous answer (0.017mM NH3 per sec) You can use use stoichiometries to convert rates Unique Average Rate • Because it can be confusing to express rates of different products or reactants, we use the Unique Average Rate in many instances aA + bB --> cC + dD The Unique Average Rate = 1 [A] 1 [B] 1 [C] 1 [D] a t b t c t d t Instantaneous Rate of Reaction When you measure the rate of a reaction, the rate is constantly changing as the composition of the reaction mixture changes •You want to choose 2 points that are close together so that you have a straight line The instantaneous reaction rate is the slope of a tangent line drawn to the graph of concentration versus time For most reactions, the rate decreases as the reaction proceeds (when we are plotting the concentration of reactants) Rate Laws and Reaction Order Let’s look at an experiment on the decomposition of dinitrogen pentoxide N2O5 --> 4NO2 + O2 5 different initial concentrations of N2O5, and 5 different rates Rate Laws • We could describe the rate of dinitrogen pentoxide decomposition as: Rate = k [N2O5] • We can see this experimentally. – As the [N2O5] increases, the rate increases • k is the rate constant for the reaction and if we plotted rate versus [N2O5], it is equal to the slope. Rate Laws Rate of N2O5 decomposition = k [N2O5] This is a rate law • An expression for the instantaneous reaction rate as a function of concentration Every chemical reaction has its own rate law and unique rate constant, k The rate constant is independent of reactant concentration but dependent on temperature Order of Reaction If we look at another reaction, we’ll find that there may be a different dependence on reactant concentration: 2NO2 (g) --> 2NO (g) + O2 (g) Rate vs. [NO2] Note shape of plot! Rate vs. [NO2]2 Note shape of plot! Order of Reaction 2NO2 (g) --> 2NO (g) + O2 (g) This observation of concentration dependence on rate is telling us something that the balanced chemical equation was and we may have missed… O=N=O O=N + + O=N=O O=N + O=O In order for the reaction to proceed, we need 2 molecules of O=N=O The rate law is: Rate of Consumption of NO2 = k [NO2]2 The reaction is a second order reaction Rate Laws and Reaction Order aA + bB --> cC + dD Rate = Rate constant [concentration of reactant A]a Or Rate = Rate constant [concentration of reactant B]b Examples: Rate = k [N2O5] (First Order) Rate = k [NO2]2 (Second Order) Doubling the [reactant] of a first order reaction doubles the reaction rate Doubling the [reactant] of a second order reaction quadruples the reaction rate Zero Order Reactions Some reactions just happen and continue at a constant rate until the reactant is completely consumed 2NH3 --> N2 + 3H2 The decomposition of ammonia is an example of a zero order reaction Experimental data tells us the order of the reaction Rate Laws and Order The rate law for a reaction is experimentally determined and cannot be known simply by looking at the balanced chemical equation for the reaction. Overall Reaction Order •We have discussed reaction order with respect to a single reactant, but we must also be able to describe the rate law for reactions that depend on more than one reactant •Anabolic reactions or synthetic reactions •To describe a reaction like this, we have to include the concentration of both reactants in the rate law Example: Overall Reaction Order • Let’s look at the reaction between: Ca(OH)2 (aq) + 2HCl (aq) CaCl2 (s) + 2H2O (l) • Because we are making solid CaCl2, the rate depends on the concentration of Ca2+ and Cl-. Rate of Consumption of Ca(OH)2 = k[Ca(OH)2][HCl]2 And the overall reaction order is the sum of the exponents = 1+2 = 3. Odd Rate Laws • • Experiments have provided data for reactions that have nonstandard reaction orders. These non-standard orders tell us specific things about the reaction 1) 2O3 (g) --> 3O2 (g) The presence of O2 slows the reaction down significantly 2) 3SO2 (g) + O2 (g) --> 3SO3 (g) [O 3 ]2 Rate = k = k [O 3 ]2 [O 2 ]1 3 [O 2 ] Rate = k [SO 2 ] [SO 3 ] 1 2 = k [SO 2 ] [SO 3 ] 1 2 Integrated Rate Laws • A rate law by itself is interesting , but not terribly informative • To make the laws useful, we need them to tell us the concentration of reactants or products at any time after the reaction begins • An Integrated Rate Law does this (I guess) Integrated Rate Law for a Zero Order Reaction • We want to find the difference in the concentration of reactant A from its initial value, [A]0 • We know that this difference will be proportional to the time elapsed since the reaction started. – Why? – The rate constant is the proportionality constant [A]0 - [A]t = kt or [A]t = [A]0 - kt Integrated Rate Laws for First Order Reactions [A] t ln = - kt [A] 0 (for when you want k) [A] t = [A] 0 e -kt (for when you are given [A] 0 and want [A] t ) Exponential decay plot The rate decreases as more and more reactant is used up Confirming that a Reaction is First Order In order to confirm that a reaction is truly first order, we need to plot ln[A]t as a function of t (does everyone understand what I mean by that?) [A] t = [A] 0 e -kt ln[A] t = ln[A] 0 - kt y = b + mx We should see a straight line! Half Lives of First Order Reactions • The half life, t1/2, of a substance is the time needed for its concentration to decrease by one half. • We use half lives in: – – – – Environmental Chemistry Nuclear Science Biochemistry Pharmacology Half Lives of First Order Reactions • The higher the value of k, the higher the rate. – The amount of time necessary to decrease the reactant concentration by 1/2 is inversely proportional to k ln2 t1/2 k The half life for first order reactions is constant Second Order Integrated Rate Laws Remember: The rate law for a second order reaction is Rate of consumption of A = k[A]2 The Integrated Rate Law is: 1 1 kt [A] t [A] 0 When we know [A]t and [A]0 or [A] 0 [A] t 1 kt[A] 0 When we know [A]0 and want [A]t