Iron Deficiency Anaemia: new entities
advertisement

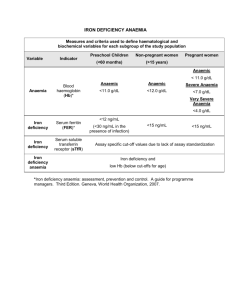
Genetic Forms of Iron Deficiency Anaemia Photis Beris, MD Professor of Clinical Haematology Department of Internal Medicine Geneva University Hospital Geneva, Switzerland Slide 1 of 21 Genetic Forms of Iron Deficiency Anaemia • Mutations in the gene encoding DMT1 • Mutations in the gene encoding glutaredoxin 5 • Hypotransferrinaemia or atransferrinaemia • Deficiency of ceruloplasmin • IRIDA (Iron-Refractory, Iron-Deficiency Anaemia) Slide 2 of 21 Iron Deficiency Anaemia Overview of Iron Homeostasis With permission from Andrews NC. Blood. 2008;112:219-230. Slide 3 of 21 Genes Involved in Hereditary Iron Deficiency Anaemia Protein (Gene Symbol) Chromosome Protein Function DMT1 (SLC11A2)1 121 Transmembrane iron transporter1 Autosomal recessive hypochromic, microcytic anaemia with hepatic iron overload1 Glutaredoxin 5 (GLRX5)1 141 Participates in iron-sulfur cluster biogenesis1 Anaemia with iron overload and sideroblasts1 Transferrin (TF)1 31 Plasma iron binding protein; ligand for TFR1 & TFR21 Iron deficiency anaemia with tissue iron overload1 Ceruloplasmin (CP)1 31 Plasma ferroxidase1 Mild iron deficiency anaemia associated with iron accumulation in the liver and brain1 Matriptase -2 (TMPRSS6)1 222 Regulates hepcidin expression by an unknown mechanism1 IRIDA1 With permission from Andrews NC. Blood. 2008;112:219-230. 1. Andrews NC. Blood. 2008;112:219-230. 2. Finberg KE, et al. Nat Genet. 2008;40:569-571. Disease Caused by Mutations Slide 4 of 21 Mutations in the Gene Encoding DMT1 Haematologic and Biologic Data of the 3 Described Families with Microcytic Anaemia Secondary to DMT1 Mutations French1 Czech2 Italian3 Age (years) 6 20 5 Hb (g/L) 75 74 85 MCV (fL) 53 54 51 MCHC (g/L) 287 296 295 Serum iron (µM/L) 28 43 36.5 Transferrin saturation (%) 68 84 90 Ferritin µg/L 18 153 34 MCV = mean corpuscular volume; MCHC = mean corpuscular haemoglobin concentration. 1. Beaumont C, et al. Blood. 2006;107:4168-4170. 2. Priwitzerova M, et al. Blood. 2004;103:3991-3992. 3. Iolascon A, et al. Blood. 2006;107:349-354. Slide 5 of 21 DMT1 Mutations Lead to Microcytic Anaemia, Low (Normal) Ferritinaemia, and Liver Iron Overload Iron absorption in the duodenum continues because the absorption of heme iron is not disturbed. In fact, in meat-eating humans it is estimated that about 2/3 of absorbed iron comes from heme. Thus, in humans, a mutation in DMT1 protein may primarily affect iron utilisation and not absorption, leading to severe microcytic iron deficiency anaemia with increased iron stores. Mims MP, et al. Blood. 2005;105:1337-1342. Slide 6 of 21 Mutations in the Gene Encoding Glutaredoxin 5 Non-erythroid cells Case Report 44-year-old man Hb 88.6g/L, MCV 59fL, TF% 52 Ferritin 1100 mg/L BM: erythroid hyperplasia, 28% ringed sideroblasts Erythroid cells The patient had a homozygous mutation that interferes with intron 1 splicing and drastically reduces glutaredoxin (GLRX5) RNA GLRX5 has an essential role in the synthesis of Fe/S clusters DFO = desferrioxamine; hb = haemoglobin; MCV = mean corpuscular volume; BM = bone marrow; Fe/S = ironsulfur. With permission from Camaschella C, et al. Blood. 2007;110:1353-1358. Slide 7 of 21 Hypotransferrinaemia or Atransferrinaemia Caucasian woman 20 years of age with: • Hb 106 g/L; MCV 75.3 fL; iron serum 15 µg/dL; serum total iron binding capacity 23 µg/dL; ferritin >2500 µg/L • Liver biopsy marked haemosiderosis; LIC 37 mg/g dry weight • DNA sequencing revealed a 10 bp deletion followed by 9-bp insertion (maternal) and a Ala >Pro mutation at position 477 (paternal chromosome) • Treatment consisted of monthly plasma infusions 500 mL + bleeding. 10 years later, Hb 120g/L; ferritin: normal Beutler E, et al. Blood. 2000;96:4071-4074. Slide 8 of 21 Hypotransferrinaemia or Atransferrinaemia Pathophysiology • Atransferrinaemia results in reduced delivery of iron to the erythroblasts and development of iron deficiency anaemia (hypochromic–microcytic). This triggers a massive but futile iron absorption leading to iron overload outside of the erythron1 • Hereditary atransferrinaemia has been reported in 7 families2 1. 2. Andrews NC. Blood. 2008;112:219-230. Beutler E, et al. Blood. 2000;96:4071-4074. Slide 9 of 21 Deficiency of Ceruloplasmin Case Presentation • 56-year-old Italian man • Hb 98 g/L; MCV 62.5 fl; MCHC 307 g/L • Serum iron 2.96 µmol/L; ferritin 423 µg/L • Ceruloplasmin 0.2 ng/mL • Insulin-dependent diabetes mellitus since the age of 40 • CRP, fibrinogen: no evidence of inflammatory disease • Liver biopsy showed iron overload • DNA sequencing of CP gene: Gly >Arg at codon 631 (homozygous state) Hb = haemoglobin; MCV = mean corpuscular volume; MCHC = mean corpuscular haemoglobin concentration; CRP = C-reactive protein. DiRaimondo D, et al. Intern Emerg Med. 2008 Apr 12. [Epub ahead of print] PMID: 18408989 Slide 10 of 21 Deficiency of Ceruloplasmin • Ceruloplasmin (CP), a copper-containing ferroxidase, cooperates to export iron with ferroportin 11 • CP deficiency results in low serum iron (= iron deficiency anaemia) with concomitant deposition in the brain, liver, pancreas, basal ganglia, and other organs1,2 • The constant feature of aceruloplasminaemia is a moderate degree of anaemia, associated with low serum iron and high ferritin1 • Differential diagnosis1 – Anaemia of chronic diseases – Wilson’s disease – Hypotransferrinaemia or atransferrinaemia • Treatment: chelation therapy; oral zinc sulfate3; no benefit from phlebotomy1 1. DiRaimond D, et al. Intern Emerg Med. 2008 Apr 12. [Epub ahead of print] PMID: 18408989. 2. Miyajima H. Neuropathology. 2003;23:345-350. 3. Kuhn J et al. Brain Dev. 2007;29:450-453. Slide 11 of 21 IRIDA (Iron-Refractory, Iron-Deficiency Anaemia) The Mask Mouse “…a chronically iron-deficient mouse with an unusual pattern of hair loss over the trunk but not the head (the mask phenotype) due to a homozygous recessive genetic mutation. Mask mice were shown to express inappropriately high levels of hepcidin mRNA in the liver, even when fed an iron-deficient diet. Using positional cloning techniques, Dr. Beutler’s group was able to ascribe the mask phenotype to a splicing error in the TMPRSS6 gene, which encodes a membrane-bound serine protease.”1,2 1. Coghill JM, Ma A. Available at: http://www.hematology.org/client_files/meeting/2007/newsdaily/HepcidinAdventureEarnsRaveReviewsatPlenary.pdf 2. Du X, et al. Science. 2008;320:1088-1092. Slide 12 of 21 Mutation in TMPRSS6 (Matriptase-2), Suppressor of Hepcidin Gene Expression, in Familial Iron Deficiency Anaemia Photograph: With permission from Du X. Science. 2008;320:1088-1092. Graphic (top right): With permission from Ramsay AJ, et al. Front Biosci. 2008;13:569-579. Graphic (bottom): With permission from Finberg KE, et al. Nat Genet. 2008;40:569-571. Slide 13 of 21 IRIDA (Iron-Refractory, Iron-Deficiency Anaemia) Generation of mutant mice TMPRSS6-/- provided evidence that matriptase-2 is an essential regulator of iron homeostasis. In fact, in mice as well as in humans, mutations in the TMPRSS6-/gene lead to severe iron deficiency anaemia. This state is characterized by reduced ferroportin expression (shown in the mice model) and both animals and humans have high hepcidin levels. Folgueras AR, et al. Blood. 2008 Jun 3. [Epub ahead of print] PMID: 18523150. Slide 14 of 21 IRIDA (Iron-Refractory, Iron-Deficiency Anaemia) Case-Reports Patient 11 Patient 22 Patient 33 Patient 43 Patient 53 Patient 63 Patient 73 Patient 83 Patient 93 Age (y) 14 18 6 13mo. 17mo. 11 7 3 15mo. Hb (g/L) 86 100 88 92 70 82 75 97 79 MCV (fL) 54.3 61 58 65 49 56 49 61 53 2.44 2.96 Ferritin (µg/L) 9 53 Transferrin saturation (%) 6.52 sTfR (mg/L) 5 2 10 5 3 4 4 2 70 133.5 IRON STATUS Fe (µmol/L) pHepcidin (ng/mL) 1. Guillem F, Blood 2008 Jul 2. [Epub ahead of print] PMID: 18596229. 2. Melis MA, et al. Haematologica. 2008 Jul 4. [Epub ahead of print] PMID: 18603562. 3. Finberg KE, et al. Nat Genet. 2008 May;40(5):569-71. Slide 15 of 21 IRIDA (Iron-Refractory, Iron-Deficiency Anaemia) Case-Reports Treatment • Oral iron administration is ineffective • Response to parenteral iron administration is partial • Anaemia becomes less severe in adulthood as a consequence of the greater availability of the limited amount of available iron to erythropoiesis Melis MA, et al. Haematologica. 2008 Jul 4. [Epub ahead of print] PMID: 18603562. Slide 16 of 21 Differential Diagnosis of Microcytic Anaemia • Thalassaemia syndromes • Certain haemoglobinopathies (Hb C) • True (classical) iron deficiency secondary to blood loss, iron-poor diet, increased iron needs, Helicobacter pylori infection or gastric pathology • Anaemia of chronic inflammatory diseases • Certain forms of sideroblastic anaemia • Genetic forms of iron deficiency anaemia Slide 17 of 21 Diagnostic Approach—Genetic Forms of Iron Deficiency Anaemia • Look for consanguinity (recessive transmission) • Is the anaemia associated with massive iron accumulation? If yes, then do detailed evaluation of CNS to distinguish between aceruloplasmia (CNS damage present) and the other forms with iron overload • If presence of basophilic stippling and double erythroid population in peripheral blood, then do bone marrow aspiration to look for the presence of ring sideroblasts Slide 18 of 21 Diagnostic Approach—Genetic Form of Iron Deficiency Anaemia • Was the anaemia detected at birth or early in life? If yes, then look for a mutation in the DMT1 or the TMPRSS6 gene • Does the patient have microcytosis with high serum iron values? If yes, then a DMT1 mutation is highly suspected • If no, then look for a TMPRSS6 mutation. Hepcidin levels are also very helpful as hepcidin is normal or increased in the TMPRSS6 mutation despite low serum iron Slide 19 of 21 Main Biologic and Clinical Differences in Genetic Forms of Iron Deficiency Anaemia DMT1 Glutaredoxin 5 Atransferrinaemia Aceruloplasminaemia TMPRSS6 (matriptase-2) At birth Usually midlife Late onset provided some transferrin is present Late onset with moderate anaemia 18–24 mo Liver iron overload Yes Yes Yes Yes No Brain damage No No No Yes No Serum iron High High Low Low Low Transferrin saturation High High High or nonmeasureable Low Low Ringed sideroblasts No Yes No No No Hepcidin levels Low Not yet measured Not yet measured Not yet measured High for low iron values Low or normal High High High Normal Age at diagnosis Ferritin Slide 20 of 21 Conclusions • Iron deficiency anaemia is an acquired disease with an estimated 3 billion people affected and represents a major public health problem worldwide1 • Recent advances in iron metabolism led to the recognition of new entities of iron deficiency anaemia in nonbleeding and “high cost diet” nourished individuals • Apparently rare, these genetic forms of iron deficiency anaemia should be recognized by haematologists, as they are refractory to classical oral or intravenous iron administration 1. Andrews NC. Blood. 2008;112:219-230. Slide 21 of 21