Enzyme-Controlled Reactions
advertisement

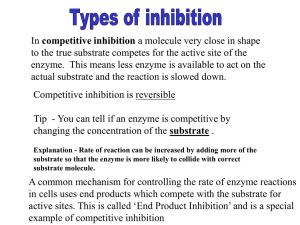
Factors Affecting the Rate of Enzyme-Controlled Reactions In this extract from an undergraduate essay, general academic words, from the Academic Word List, are highlighted in bold. It is important that you understand these words and can use them. Study the words in bold carefully. Learn them. Enzymes are proteins, or occasionally RNA, found in cells that act as biological catalysts. Their mode of action is to lower the activation energy of the reaction by forming an enzyme-substrate complex, which is a more stable intermediate than would be formed, were the enzyme not present. The fact that the activation energy is lower is the greatest effect of the catalyst as it allows the reaction to occur, at the rates required, in the relatively cool conditions within the cells. The enzymes can do this in one, or more, of three ways. These are as follows: Firstly, they can bring two reactants into close enough proximity to allow the reaction to occur more readily than if the reaction relied solely on the reactants moving randomly around the cell, and hopefully “bumping” into each other. Secondly, being proteins and therefore made up from many different amino acids, the enzymes can have varying charges in different parts of the molecule. This can lead to the substrates becoming charged, in such a way that the reaction occurs more readily. The final method of enzyme action is to force the substrate into an unusual shape, which puts stresses on the bonds within the molecule. These stresses make it easier to break the required bonds, and therefore easier for the reaction to occur. The rates of all of these methods of action are affected by external factors. Two of these are the concentration of the enzyme itself, and the concentration of the substrates. The concentration of the enzyme is far more often a limiting factor than the substrate concentration. Other factors also affect the rate of enzymic reactions; one of the major influences is temperature. Enzymes, being made, largely, of proteins are affected by heat in the same way as other proteins, that is they are denatured at high temperatures. However, higher temperatures, in moderation, have a positive effect on the rate of the reaction. This is because higher temperatures increase the kinetic energy of the enzyme and substrates, meaning that they will move faster, and so the time it takes for the correct “parts” to collide will be shortened. This will have a particular effect when neither substrate nor enzyme are limiting, i.e. when it is the diffusion time that is limiting the rate of reaction. Therefore temperature has two effects on the rate of reaction: up to a point it speeds up the rate of reaction, by increasing the chance of reactants “meeting” each other, in the presence of an enzyme, and therefore increasing the rate adapted with permission: Sandra Haywood, University of Nottingham of reaction; however, beyond this point, the enzymes are denatured, leading to the rate of reaction decreasing. Another factor that affects rate of reaction is the pH of the cytoplasm or solution the enzymes are to be found in. This affects the rate of reaction because the shape of the enzymes is altered by the change in pH as a consequence of variations in the configuration of the weak non-covalent bonds, because of changes in the ionic and hydrophobic interactions within the protein structure. This in turn means that some of the enzymes no longer fit their substrate as they should, hence making the reaction happen much more slowly, or not at all. Another influence on the rate of enzymic reactions is the presence, or absence, of inhibitory substances. Inhibition can occur in one of two ways, firstly reversible inhibition, and secondly irreversible inhibition. Reversible inhibition occurs when the inhibitor closely resembles the substrate, and so it can fit in the active site of the enzyme, i.e. it competes with the substrate. An example of this is malate as an inhibitor for succinate dehydrogenase. This sort of inhibition is affected by the relative concentrations of the inhibitor and substrate, so that at very high substrate concentrations, the inhibitor cannot bind to the active sites, has very little effect, and eventually leaves the body. Irreversible inhibition does not work in this way. Instead the enzyme has a second site where the inhibitor can bind. This binding then leads to the active site changing shape, and therefore not allowing the substrate to bind with it. This sort of inhibition cannot be affected by the relative concentrations of substrate and inhibitor as they bind to different sites. It is called irreversible, because once bound the inhibitor is very hard to remove, hence the inhibition is not reversible. All these factors affect the rate of enzymic reactions, and so it is a combination of all these factors that finally determines the rate of reaction. Original text: extract from undergraduate essay by Joanna Haywood adapted with permission: Sandra Haywood, University of Nottingham