Charges Assignment
advertisement
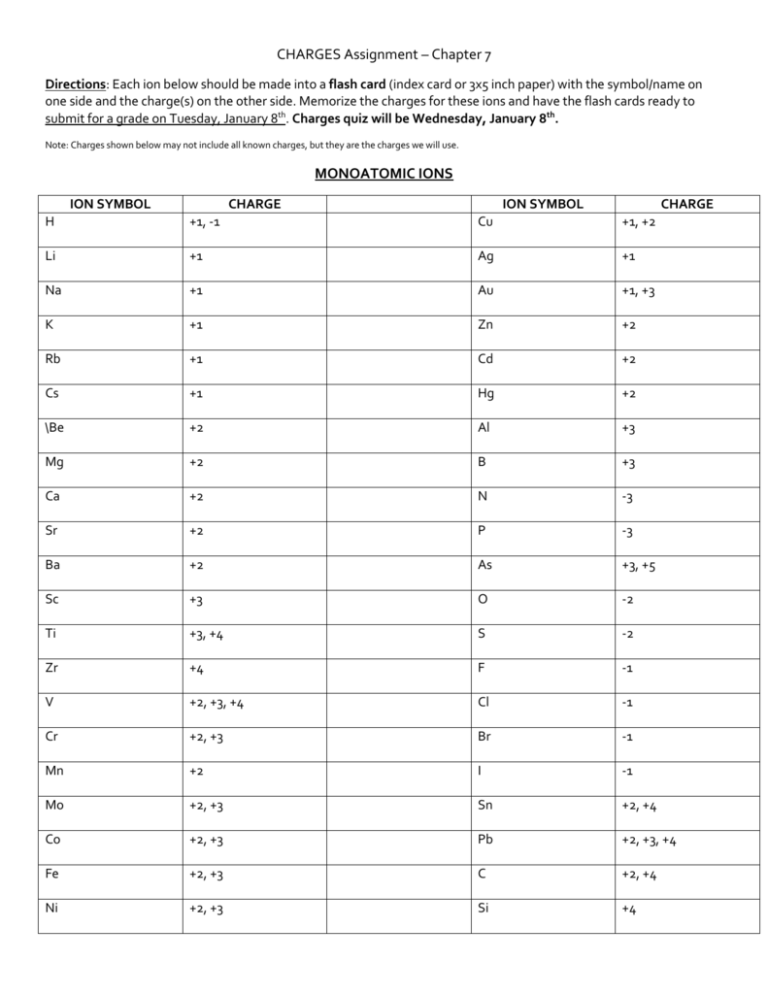
CHARGES Assignment – Chapter 7 Directions: Each ion below should be made into a flash card (index card or 3x5 inch paper) with the symbol/name on one side and the charge(s) on the other side. Memorize the charges for these ions and have the flash cards ready to submit for a grade on Tuesday, January 8th. Charges quiz will be Wednesday, January 8th. Note: Charges shown below may not include all known charges, but they are the charges we will use. MONOATOMIC IONS ION SYMBOL CHARGE ION SYMBOL CHARGE H +1, -1 Cu +1, +2 Li +1 Ag +1 Na +1 Au +1, +3 K +1 Zn +2 Rb +1 Cd +2 Cs +1 Hg +2 \Be +2 Al +3 Mg +2 B +3 Ca +2 N -3 Sr +2 P -3 Ba +2 As +3, +5 Sc +3 O -2 Ti +3, +4 S -2 Zr +4 F -1 V +2, +3, +4 Cl -1 Cr +2, +3 Br -1 Mn +2 I -1 Mo +2, +3 Sn +2, +4 Co +2, +3 Pb +2, +3, +4 Fe +2, +3 C +2, +4 Ni +2, +3 Si +4 POLYATOMIC IONS NAME Ammonium ION NH4 Dimercury Hg2 CHARGE +1 NAME Carbonate ION CHARGE CO3 -2 +2 Chromate CrO4 -2 DiChromate Cr2O7 -2 Acetate CH3COO -1 Oxalate C2O4 -2 Perchlorate ClO4 -1 Peroxide O2 -2 Chlorate ClO3 -1 Sulfate SO4 -2 Chlorite ClO2 -1 Sulfite SO3 -2 Hypochlorite ClO -1 Cyanide CN -1 Hydroxide OH -1 Arsenate AsO4 -3 Nitrate NO3 -1 Phosphate PO4 -3 Nitrite NO2 -1 Phosphite PO3 -3 Permanganate MnO4 -1