Cellular Chemistry
advertisement

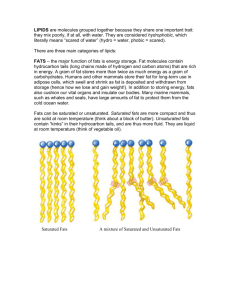
CELLULAR CHEMISTRY Chemicals Matter—You’re Made of Them! By: Heidi Hisrich Examination • Look at your hand. What do you see? What if you zoomed in? What’s the smallest thing you could see? • Your hand is made of cells, which are made of molecules, which are made of atoms. But where did they all come from? How did they get there? Food! • We are, quite literally, what we eat! • Almost every atom in every molecule in every cell in our bodies came from food (a little bit came from water and air), so what we eat IS matter AND matters! Molecules • And the food we eat is made of molecules • A molecule is a group of atoms that are stuck together and most atoms are found in molecules The 4 Types of Macromolecules • There are 4 main kinds of molecules (besides water) in the foods that we eat. They are all ORGANIC (alive). What are they? • Carbohydrates • Lipids • Proteins • Nucleic Acids Nucleic Acids • Found in ALL living things—allow them to pass on traits from parents to offspring (heredity) • There are 2 kinds. What are they? RNA (ribonucleic acid) DNA (deoxyribonucleic acid) RNA Single stranded Sugar is ribose Bases are A, C, G & U DNA Double stranded Sugar is deoxyribose Bases are A, C, G & T Function Store genetic info (DNA) and transcribe and translate it into protein (RNA) Monomer that makes them up nucleotides Elements found in them • Oxygen • Hydrogen • Carbon • Phosphorus • Nitrogen Foods they’re in? E V E R Y T H I N G ! Proteins • What our bodies are mostly MADE OF—we are made of protein, just like the animals we eat for protein are (cows, chickens, fish, etc). • What is the building block (monomer) that makes them up? Sketch and label the parts How many are there? Only 20 in humans, but they make up MILLIONS of different proteins! Amino Acids Elements in proteins Carbon Hydrogen Nitrogen Oxygen What do they look like? Most complicated type of macromolecule BY FAR!!! Examples of Proteins Keratin collagen Indicator • Biuret Protein-Heavy Foods From animal sources From vegetable sources 4 Types of Proteins • Transporting—hemoglobin moves oxygen • Structural—collagen makes up skin • Enzymatic—amylase breaks down starch • Signaling—insulin tells cells to take in sugar Lipids (fats) • Several kinds • Unsaturated • Saturated • Trans fats Which are healthiest? Least healthy?? HEALTHIEST—unsaturated LESS HEALTHY—saturated THE WORST!!!—trans fats What it looks like • Look for zig-zag TAILS (fatty acid chains) Unsaturated Fats • NOT saturated in hydrogen (at least one double bond) Saturated Fats • SATURATED in hydrogen (no double bonds in chains) Trans Fats Created in a LABORATORY—chemists turn liquid fats into more stable solid fats Unsaturated fats in foods • Usually LIQUID at room temp • Mostly from VEGETABLE SOURCES Saturated fats in foods • Usually SOLID at room temp • Mostly from ANIMAL SOURCES (less legs is better!) Trans fats in food Look on label for “partially hydrogenated oils”—0 g trans fats does NOT mean NO trans trans fats!!! Elements in Lipids Hydrogen Oxygen Carbon Function of Lipids • Part of cell membrane • Needed to make hormones • Provide long term energy storage Indicator Carbohydrates • Referred to as “carbs”—their ONLY job is to give you energy—can be small/simple or much more complex Monosaccharides (simple sugars) • “one sugar”—each is a SINGLE RING Examples of monosaccharides GLUCOSE FRUCTOSE Indicator Benedict’s Dissacharides • “Two sugars”—double rings