Diffusion and Osmosis PP
advertisement

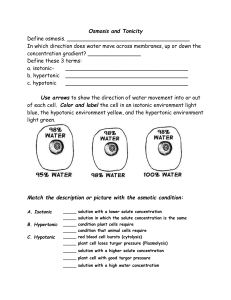
HIGH Concentration LOW Concentration High Concentration LOW Concentration HIGH Concentration LOW Concentration Diffusion and osmosis Types of Cellular Transport How “stuff” gets into and outside of the cell membrane Passive Transport cell does NOT USE ENERGY 1. Diffusion 2. Facilitated Diffusion 3. Osmosis high Active Transport cell USES ENERGY 1. Protein Pumps 2. Endocytosis 3. Exocytosis Weeee!!! low This is gonna be hard work!! high low Bacon Frying… What do you smell? Why do you smell this? Bacon Frying… • Fat droplets are released into the air by the steam coming off of the bacon • Causes the fat droplets (particles) to collide and disperse. In science terms… • DIFFUSION IS OCCURING! The bacon fat droplets (particles) diffuse from the area of high concentration (directly above the frying pan), to areas of low concentration (the room) Continues until equilibrium is reached Particles equally distributed throughout the space (room) In the cell this is called HOMEOSTASIS!! Diffusion • Diffusion - Defined as the movement of particles from areas of high concentration to areas of low concentration (down the concentration gradient) – Particles spread out – Ex: Solute = perfume Solvent = the air Low Solute Concentration High Solute Concentration Diffusion What Can go through? Oxygen Carbon dioxide What 3 things effect the rate of diffusion? (how fast diffusion occurs) Diffusion rate (how fast it occurs) depends on: 1. Size- smaller molecules move more easily Concentration- 2. high concentration = faster spread Ex: The more bacon you have (=more fat droplets released), the faster the smell will spread throughout the room Temperature- 3. high temps speed up the process Which will cause the smell to disperse faster? Cold bacon in a frying pan and the stove is OFF OR Bacon in a frying pan and the stove in ON What is a semi-permeable membrane? • A membrane that allows certain molecules to pass through it by diffusion or osmosis • Think about a Ping-Pong net.. • Which would pass through? Cell membranes are semi-permeable • Water and some gases move through the membrane easily, while larger molecules like proteins and sugars do not. • Cells must regulate- molecules move from internal environment to external environment (and vice versa) so that both environments have equal concentrations. – Animation: How Osmosis Works Osmosis • Diffusion that involves water. • Movement of water across a semi-permeable membrane • WATER moves across the cell membrane • Water moves across the cell membrane from areas of low solute concentration to high solute concentration Osmosis • Diffusion that involves water. • Movement of water across a semi-permeable membrane • Water moves from areas of low solute concentration to areas of high solute concentration Why do your fingers “shrivel–up” when you are in the water? Effects of Osmosis on Life • Osmosis- diffusion of water through a selectively permeable membrane. • There are 3 types of solutions (=Tonicity) 1.) Isotonic 2.) Hypertonic 3.) Hypotonic Solution Cell Solution Cell Isotonic Solution • Solutions that contain the same concentration of solutes as the cytoplasm are called isotonic (or normal saline) solutions. • There is no net movement of water either into or out of the cell. • Cells maintain their normal shape. Isotonic Solution Isotonic The concentration of solutes in the external solution is equal to the concentration of solutes inside the cell. Result Water moves equally in both directions and the cell remains same size! (Dynamic Equilibrium) Hypertonic Solution • A hypertonic solution is a solution that contains more solutes than the cytoplasm of the cell. (hyper) • Has less water than the cell and water moves out of the cell. • The cells shrink. Hypertonic Solution Hypertonic: The solution has a higher concentration of solutes and a lower concentration of water than inside the cell. (High solute; Low water) shrinks Result: Water moves from inside the cell into the solution: Cell shrinks (Plasmolysis/crenation)! Hypotonic Solution • A hypotonic solution contains less solute (thus, more water) than the cytoplasm of the cells. • The water will move into the cells resulting in the swelling and lysis of the cells. Hypotonic Solution Hypotonic: The solution has a lower concentration of solutes and a higher concentration of water than inside the cell. (Low solute; High water) Result: Water moves from the solution to inside the cell): Cell Swells and bursts open (cytolysis)! Let’s take a closer look! https://www.youtube.com/watch?v=Ia Z8MtF3C6M What type of solution are these cells in? A B C What type of solution are these cells in? A Hypertonic B Isotonic C Hypotonic Tonicity Used to compare different solutions Hypertonic- higher solute concentration relative to another *think hyperactive Hypotonic- lower solute concentration relative to another *think hypothermia Isotonic- equal solute concentrations between two solutions ENVIRONMENT 10% NaCL 90% H2O CELL 10% NaCL 90% H2O cell is at _______________. WhatThe is the direction of water movement? 39 Cell in Isotonic Solution ENVIRONMENT 10% NaCL 90% H2O CELL 10% NaCL 90% H2O equilibrium cell is at _______________. WhatThe is the direction of water movement? NO NET MOVEMENT 40 10% NaCL 90% H2O CELL 20% NaCL 80% H2O What is the direction of water movement?? 41 Cell in Hypotonic Solution 10% NaCL 90% H2O CELL 20% NaCL 80% H2O The cell is HYPERTONIC and the solution is HYPOTONIC 42 15% NaCL 85% H2O ENVIRONMENT CELL 5% NaCL 95% H2O What is the direction of water movement? 43 Cell in Hypertonic Solution 15% NaCL 85% H2O ENVIRONMENT CELL 5% NaCL 95% H2O The cell is HYPOTONIC and the solution is HYPERTONIC 44 Cells in Solutions 45 Isotonic Solution NO NET MOVEMENT OF H2O (equal amounts entering & leaving) Hypotonic Solution CYTOLYSIS Hypertonic Solution PLASMOLYSIS 46 Cytolysis & Plasmolysis Cytolysis Plasmolysis 47 Osmosis in Red Blood Cells Isotonic Hypotonic Hypertonic 48 What Happens to Blood Cells? 49