chapter five notes97-2002rev
advertisement

TYPES OF COMPOUNDS
Chemical Family Resemblances
Binary salts
Binary salts are made of a metal and a
nonmetal – only two different elements.
Examples: NaCl, MnO2
Binary salts are named with the name of the
metal first, then the name of the nonmetal
with the “-ide” ending.
Example: K2O
potassium oxide
FORMULAS
The
formula unit is the simplest ratio of
ions in the salt.
Ga2O3
2:3 ratio of gallium atoms to oxygen atoms
2 gallium atoms and 3 oxygen atoms make
one formula unit
formulas
Electrons
and charge are conserved in a
formula unit.
– 2 gallium atoms have a total of 6 valence
electrons and no charge
– 3 oxygen atoms have a total of 18 valence
electrons and no charge
– so gallium oxide (Ga2O3) has 18+6=24
valence electrons and no charge
conservation
Conservation
of electrons and charge in
gallium sulfide (Ga2S3)
conservation
oxidation numbers
Oxidation
number of an ion is equal to
the charge on an ion after it gains or
loses electrons.
All atoms gain or lose electrons to try to
attain a noble gas configuration (8
valence electrons)
Noble gases have no oxidation numbers
oxidation numbers
Metals
– lose all valence electrons,
positive (+) oxidation numbers
Metals lose electrons so as to expose full
valence shell in next lower level
– Alkali metals and hydrogen are +1
– Alkaline earths are +2
– Aluminum and friends are +3
oxidation states
– Tin and lead are +2 or +4
– Transition metals vary
Nonmetals
– gain electrons, negative (-)
oxidation numbers
Enough electrons are gained to complete
the valence shell
– Oxygen is always –2, and sulfur is –2 unless
with oxygen
ternary salts
– Halogens are –1 unless with oxygen
– Nitrogen and phosphorus are –3 unless with
oxygen or halogens
Ternary
salts are composed of more than
two elements
Ternary salts contain polyatomic ions
– Polyatomic ions contain more than one atom
example: CO3-2 carbonate
polyatomic ions
Polyatomic
anions have a (-) charge, and
polyatomic cations a (+) charge
Polyatomic ions act as a unit – the
subscripts of the formula may not be
changed
Names and formulas
– Most names end in “-ate” or “-ite”, which
means the ion contains oxygen
naming polyatomic ions
– Examples: sulfate (SO4-2), sulfite (SO3-2)
– The ending and prefix (if present) indicate
the relative number of oxygen atoms in the
formula.
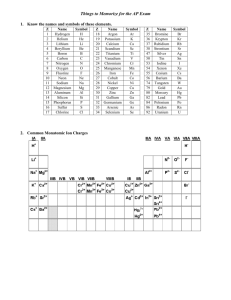
perchlorate
chlorate
chlorite
hypochlorite
ClO4–
ClO3–
ClO2–
ClO–
polyatomic cations
The
“-ium” ending means a positive ion
(hydronium, H3O+, and ammonium,
NH4+)
Multiple ions are indicated by
parentheses and a subscript
– Example: magnesium hydroxide is
Mg(OH)2
– Ammonium sulfide: (NH4)2S
formulas with
polyatomic ions
Formulas
are made the same way as the
binary salts, with the criss-cross method
+
Na 2
CO3
-2
+2 (OH-)
CaCa
2
Naming ternary salts
Ternary
salts are named with the metal
name first, then the name of the
polyatomic ion
K3PO4
potassium phosphate
Transition metal salts
Many
transition and “other” metals have
more than one oxidation number
These numbers are found on some
periodic tables
Metals to know: Fe (+2, +3), Cu (+1, +2),
Ag (+1), Zn (+2), Sn (+2, +4), Pb (+2, +4),
Bi (+3, +5)
transition metal salts
Oxidation
number of transition metal is
indicated by a Roman numeral in
parentheses
FeCl3 is iron (III) chloride
Name these: CrO chromium (II) oxide
Cr2O3
chromium (III) oxide
CrO3
chromium (VI) oxide
transition metal salts
The
Roman numeral is not needed if
there is only one oxidation state for the
metal (i.e. Zn, Ag, Sc)
The Roman numeral is also used for
“other” metal salts like tin (II) fluoride
(SnF2, formerly used in toothpaste)
Transition metal salts are often brightly
colored
hydrates
Hydrates
are salts that have water
incorporated into the crystal structure
The water is usually associated with the
cation
The number of water molecules in the
crystal are specified in the formula
MgCl2. 6H2O
hydrates
The
dot means they are not chemically
bonded
Names of hydrates – “hydrate” plus a
prefix is added to the salt name
MgCl2. 6H2O
is magnesium chloride hexahydrate
Prefix indicates the number of water
molecules
hydrate prefixes
mono = 1
tri = 3
penta = 5
hepta = 7
nona = 9
di = 2
tetra = 4
hexa = 6
octa = 8
deca = 10
Formation of hydrates
Hydrates
can be formed when certain
salts are crystallized from water.
Example – CuSO4. 5H2O {copper (II)
sulfate pentahydrate}
Hygroscopic compounds become
hydrates by taking water from the air.
Formation of Hydrates
Example
– sodium carbonate becomes
sodium carbonate decahydrate
(Na2CO3. 10H2O)
Deliquescent compounds take enough
water from the air to form concentrated
solutions – examples: calcium chloride
(CaCl2), sodium hydroxide (NaOH)
More about polyatomic ions
Bonding
– Polyatomic ions form ionic bonds with
metals
– They are held together with covalent bonds
Formal
oxidation states can be assigned
to each atom in the ion using the
oxidation state rules
Oxidation state rules
Oxygen
is always -2
Hydrogen is always +1
Sulfur is -2 unless with oxygen
Nitrogen and phosphorus are -3 unless
with oxygen
Halogens are -1 unless with oxygen
When with oxygen oxidation states of
other atoms vary
Oxidation states of atoms
in polyatomic ions
The
sum of all the oxidation states must
add to the charge of the ion
Carbonate CO3-2
– oxygen – always (–2) charge
– total negative charge = (-6)
overall
(+4)
charge is (-2), so carbon must be
Oxidation states of atoms
in polyatomic ions
Try
these:
Arsenate AsO4-3
O is -2, As is +5
Cyanate NCO-1
O is -2, N (not next to O) is -3, so C must
be +4
Dot structures of
polyatomic ions
All
valence electrons must be counted,
with extras added for a negative charge.
Carbonate CO3-2
Dot structures of
polyatomic ions
Make
single bonds between all atoms
Dot structures of
polyatomic ions
Pair
all electrons and make double
bonds where necessary to fulfill the octet
rule
Dot structures of
polyatomic ions
Add
brackets and the charge
Dot structures of
polyatomic ions
For
positive ions, leave out valence
electrons for positive charge
Example: Ammonium (NH4+)
has 5 + 4 -1 = 8 valence electrons
Molecular substances
Made
of molecules, which are loosely
held together
Tend to be liquids, gases or low melting
solids
Liquids can be purified by distillation
Solids can be purified by
recrystallization
Most are insulators
Molecular substances
Molecular
elements
Most nonmetals are molecular
Diatomic gases – H2, N2, O2, F2, Cl2, Br2,
I2 (BrINClHOF)
Bromine also exists as a liquid, and
iodine exists as a solid
allotropes
Many
elements exist in more than one
molecular form
oxygen (O2) and ozone (O3)
carbon:
– charcoal, soot (random arrangement)
– graphite (flat sheets)
– diamond (three dimensional crystal lattice)
allotropes
fullerenes (hollow balls)
linear acetylenic carbon
-(-CC-CC-CC-)x phosphorus (P4):
– black (three dimensional, semiconductor)
– red (concatenated P4 molecules, used in matches)
– white (individual P4 molecules, unstable in air)
Different allotropes have different properties
Formulas and names of
small molecules
Many
have common names (i.e. water,
ammonia)
Systematic names use prefixes for each
element – same set of prefixes as for
hydrates
P2O5 – diphosphorus pentoxide
N2O – dinitrogen monoxide
Formulas and names of
small molecules
“mono”
is not used for the first element
in a compound
CO2 – carbon dioxide
CO – carbon monoxide
SO3 – sulfur trioxide
CCl4 – carbon tetrachloride
Organic compounds
Covalent
carbon containing compounds
– usually also contain H; may also
contain O, N, S, halogens, P
Many names are derived from alkane
names
Alkanes are hydrocarbons (containing
only C and H) with all single bonds
alkanes
Alkanes are named for the number of carbons
in a chain
CH4 – methane
C2H6 – ethane
C3H8 – propane
C4H10 – butane
C5H12 – pentane
C6H14 – hexane
C7H16 – heptane
C8H18 – octane
C9H20 – nonane
C10H22 – decane
General formula for an alkane: CnH2n+2
alkanes
Carbon
always makes 4 bonds in organic
compounds, and hydrogen makes only 1
bond
Oxygen makes 2 bonds (two lone pairs),
and nitrogen makes three bonds (one
lone pair)