Chapter 8: Major Elements
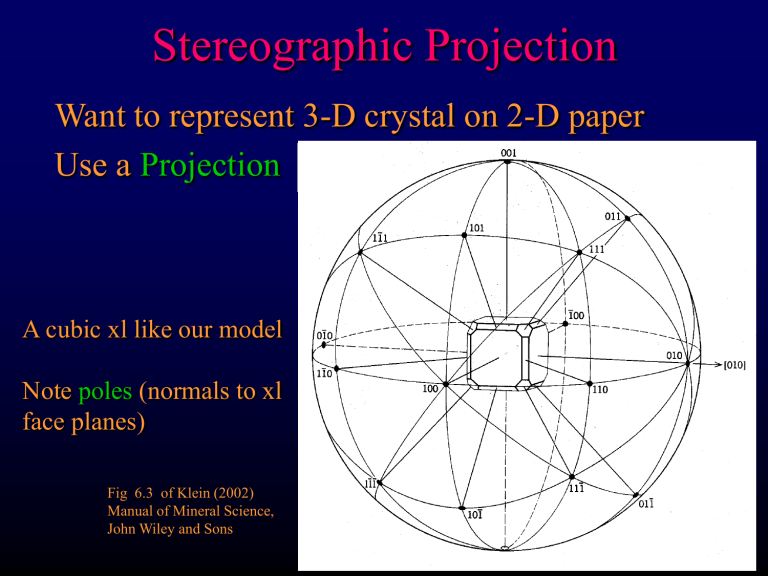
Stereographic Projection
Want to represent 3-D crystal on 2-D paper
Use a Projection
A cubic xl like our model
Note poles (normals to xl face planes)
Fig 6.3 of Klein (2002)
Manual of Mineral Science,
John Wiley and Sons
Spherical Projection
Click to run animation Case Klein animation for Mineral Science, © John Wiley & Sons
Stereographic Projection
The outer sphere is a spherical projection
Plot points where poles intersect sphere
Planes now = points
But still 3-D
Fig 6.3
Stereographic Projection
Gray plane =
Equatorial Plane
Want to use it as our 2-D representation and project our spherical poles back to it
This is a 2-D stereographic projection
Fig 6.5 of Klein (2002)
Manual of Mineral Science,
John Wiley and Sons
Stereographic Projection
D and E are spherical
D' and E' are stereographic
Distance GD' = f( r
) as r 90 D’
G as r 0 D’
O
Fig 6.6 of Klein (2002) Manual of Mineral Science, John Wiley and Sons
Stereographic Projection
We can thus use the angles and calculate the 2-D distances from the center to find the stereographic poles directly
Or we can use special graph paper and avoid the calculation
Fig 6.5 of Klein (2002)
Manual of Mineral Science,
John Wiley and Sons
Inclined Planes and Great Circles
Great Circle as stereographic projection calculated from angle r
Great circles on stereographic projection = locus of all points projected from the intercept of an inclined plane to the equatorial plane
(bowl analogy)structural geology
Use your hand for dip and a pencil for the pole of (011) at 45 o from vertical
This is the graph paper for avoiding calculating the distance from the center as a function of r each time
It is graduated in increments of 20 o
Back to Fig. 2.42
(111) (100) (111)
(011) (100) all coplanar
(= zone )
Thus all poles in a zone are on the same great circle!!
How do we find the zone axis??
Fig 6.3 of Klein (2002) Manual of Mineral Science, John Wiley & Sons
Small circles
Gives angles between any two points on a great circle
= the angle between 2 coplanar lines!!
20 o
Combines great circles and small circles in 2 o increments
The Wulff Net
Stereographic Projection
How to make a stereographic projection of our crystal
Use a contact goniometer to measure the interfacial angles (also measures normals: poles)
Fig 6.2 of Klein (2002)
Manual of Mineral Science,
John Wiley and Sons
Plot Cardboard Model
Isometric System (p. 93)
Crystallographic Axes
“The crystal forms of classes of the isometric system are referred to three axes of equal length that make right angles with each other. Because the axes are identical, they are interchangeable, and all are designated by the letter a. When properly oriented, one axis, a
1
, is horizontal and oriented front to back, a
2 vertical.” is horizontal and right to left, and a
3
+ a
1 is
90
+ a
3
90 90
+ a
2
Plot (100) (001) (010) (110) (101) (011):
= top half o = bottom half
How plot (111) ?
a) Plot (110) & then plot (111) between (110) and (001)
(110)
(111) = 36.5
o
- go in from primitive b) No measure technique:
(111) must lie between (110) & (001) (zone add rule) also between (100) & (011) thus intersection of great circles
(111)
The finished product
Fig 6.8 of Klein (2002)
Manual of Mineral Science, John
Wiley and Sons face poles and principal zones symmetry elements
Once finished can determine the angles between any 2 faces w/o measuring.
What is (100)
(111) ?
(54.5
o )
(111)
(111) ?
(70 o )
Model #75-
How can you use the position of the (111) face on a stereonet to determine: a/b?
b/c?
a/c?
Twinning
Rational symmetrically-related intergrowth
Lattices of each orientation have definite crystallographic relation to each other
Aragonite twin
Note zone at twin plane which is common to each part
Although aragonite is orthorhombic, the twin looks hexagonal due to the 120 o O-C-O angle in the CO
3 group
Redrawn from Fig 2-69 of Berry,
Mason and Dietrich, Mineralogy,
Freeman & Co.
Twinning
Twinning
Twin Operation is the symmetry operation which relates the two
(or more) parts (twin mirror, rot. axis)
1) Reflection (twin plane)
Examples: gypsum “fish-tail”, models 102, 108
2) Rotation (usually 180 o ) about an axis common to both (twin axis): normal and parallel twins.
Examples: carlsbad twin, model 103
3) Inversion (twin center)
The twin element cannot be a symmetry element of the individuals. Twin plane can't be a mirror plane of the crystal
Twin Law is a more exact description for a given type
(including operation, plane/axis, mineral…)
Contact & Penetration twins
Both are simple twins only two parts
Multiple twins (> 2 segments repeated by same law)
Cyclic twins - successive planes not parallel
Polysynthetic twins
Albite Law in plagioclase
Twinning
Mechanisms:
1) Growth
Growth increment cluster adds w/ twin orientation
Epitaxial more stable than random
Not all epitaxis
twins
Usually simple & penetration synneusis a special case
Twinning
Mechanisms:
1) Growth
Feldspars:
Plagioclase: Triclinic Albite-law-striations a-c a-c b b
Twinning
Mechanisms:
1) Growth
Feldspars:
Plagioclase: Triclinic Albite-law-striations
Twinning
Mechanisms:
2) Transformation (secondary)
SiO
2
: High T is higher symmetry cyclic twinning in inverted low quartz
High Quartz P6
2
22 Low Quartz P3
2
21
Twinning
Mechanisms:
2) Transformation (secondary twins)
Feldspars:
Orthoclase (monoclinic)
microcline (triclinic)
Monoclinic
(high-T) a-c
Triclinic
(low-T) a-c b b
Twinning
Mechanisms:
2) Transformation (secondary)
Feldspars:
K-feldspar: large K
lower T of transformation
“tartan twins”
Interpretation wrt petrology!
Twinning
Mechanisms:
3) Deformation (secondary)
Results from shear stress greater stress
gliding, and finally rupture
Also in feldspars.
Looks like transformation, but the difference in interpretation is tremendous
Mechanisms:
3) Deformation (secondary)
Results from shear stress. Plagioclase
Mechanisms:
3) Deformation (secondary)
Results from shear stress. Calcite
X-ray Crystallography
X-ray wavelengths are on the same order of magnitude as atomic spacings.
Crystals thus makes excellent diffraction gratings
Can use the geometry of the x-ray spots to determine geometry of grating (ie the crystal)
X-ray Crystallography
X-ray generation
W Cathode
(-) electrons
Cu Anode
(+)
X-rays
X-ray Crystallography
X-ray generation
Continuous & characteristic spectrum (Fig. 7.2)
I l
Continuous from E loss of collisions
Characteristic is quantized
X-ray Crystallography
Destructive and constructive interference of waves
Bragg Equation: in phase in phase x
Y q q q d
X-ray Crystallography n l
=2dsin q n is the “order”
As soon as the crystal is rotated, the beam ceases
(This is diffraction , not reflection)
Only get diffraction at certain angles!
Relation between l and d and q
Y x q q d
Methods:
X-ray Crystallography
1) Single-Crystal: Laue Method
Several directions simultaneously fulfill Bragg equations
Good for symmetry, but poor for analysis because distorted
Fig 7.39 of Klein (2002) Manual of Mineral
Science, John Wiley and Sons
Methods:
X-ray Crystallography
1) Single-Crystal: Precession
Use motors to move crystal & film to satisfy Bragg equations for different planes without distortions
Fig 7.40 of Klein (2002)
Manual of Mineral Science,
John Wiley and Sons
Methods:
X-ray Crystallography
2) Powder-
Easiest
Infinite orientations at once, so only need to vary q
Cameras and diffractometers





