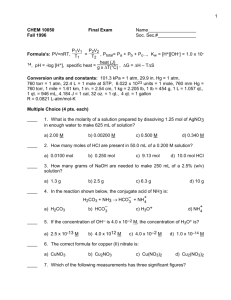
Solutions

Solutions
Composition
solute solvent dilute concentrated
Composition
Molarity (M)— the number of moles of solute per liter of solution mol/L
Composition
Mass percent— the mass of solute per mass of solution x 100 unit-less
Composition
Mole fraction ( )—
( the moles of solute per total moles of solution
solute
) or the moles of solvent per total moles of solution ( solvent
)
Composition
Molality (m)— the moles of solute per kilogram of solvent mol/kg
Composition
Normality (N)— the equivalents of solute per liter of solution eq/L
Composition
Equivalent— the mass of an acid or base that can furnish or react with 1 mole of protons
Composition
Equivalent— the mass of an oxidizing or reducing agent that can accept or furnish 1 mole of electrons
Composition Practice
Mylanta ® Liquid contains
200mg of aluminum hydroxide for every 5mL of solution.
Composition Practice
1. Assuming that the aluminum hydroxide is the only ingredient, what is the molarity of the solution?
Composition Practice
2. Assuming that the density of the solution is 1g/mL, what is the mass percent of the solution?
Composition Practice
3. Assuming that the mass of water is
4800mg, what are the mole fractions of both the solute and the solvent?
Composition Practice
4. What is the molality of the solution?
5.What is the normality of the solution?
1. 0.513 mol/L
2. 4% (mass)
3. 0.00953 =
0.990 = solute solvent
4. 0.534 mol/kg
5. 1.538 eq/L
Energy of Solution Formation
“like dissolves like” due to the energy associated with the formation of a solution
Step 1: solute expands (endo)
Step 2: solvent expands (endo)
Step 3: solute and solvent interact (usually exo)
Energy of Solution Formation sum of steps 1-3 gives the enthalpy of solution, or heat of solution ( H soln
)
H soln
= H
1
+ H
2
+ H
3
Energy of Solution Formation
Consider mixing oil and water…
They are immiscible.
Oil molecules are large and have LDF…thus, oil expansion
H
1 will be large and positive for the
Energy of Solution Formation
Water molecules have
H-bonds…thus, water expansion
H
2 will be large and positive for the
Energy of Solution Formation
H
3 will be small since nonpolar and polar molecules tend to have no attractive forces
Energy of Solution Formation
H soln because of the large impact that will be large and positive
H
1 and H
2 have.
Thus, it would require a large amount of energy for the oil and water to mix
Energy of Solution Formation
Consider mixing NaCl and water…
They are soluble.
NaCl formula units have strong ionic forces…thus,
H
1 will be large and positive for the NaCl expansion
Energy of Solution Formation
Again, water molecules have
H-bonds…thus, water expansion
H
2 will be large and positive for the
Energy of Solution Formation
H
3 will be large and negative since the attractive forces between the ions and the polar water molecules are so strong
Energy of Solution Formation
The H soln for NaCl in water is actually 3 kJ/mol. It is positive and small…and it is more disordered than the original NaCl and water… hence, favorable.
Solubility
• the amount of solute that dissolves in solvent
Factors Affecting
Solubility
• Structure
• Pressure
• Temperature
Structure’s Effect
• A, D, E, & K are fat-soluble because nonpolar, long H-C chains (hydrophobic)
• B & C are water-soluble because they have polar
0-H and C-O bonds
(hydrophilic)
Pressure’s Effect
• Gas in a liquid
• Increased pressure above a liquid means increased solubility of a gas in the liquid…Henry’s
Law
Henry’s Law
• Amount of a gas dissolved in solution is directly proportional to the pressure of the gas above the solution.
Henry’s Law
• P = kC
• S
1
P
1
= S
P
2
2
• Obeyed particularly well in non-dissociated solutions
Temperature’s Effect
• Most solids’ solubilities will increase as temperature is increased…but not all
• Must be determined by experimentation
Temperature’s Effect
Na
2
SO
4
Temperature’s Effect
• Gases’ solubilities will decrease as temperature is increased
• Thermal pollution
Lake Nyos, Cameroon
Lake Nyos, Cameroon
• August 21, 1986
• >1700 people and >3000 cattle killed
• Lake turn-over thought to have been caused by volcanic activity
Lake Nyos, Cameroon
Vapor Pressure
• decreases with the addition of a nonvolatile solute to a solvent
• François Raoult studied the effects of a solute on vapor pressure
Raoult’s Law
•P soln
= (P o solvent
)( solvent
•Linear relationship
)
• y = mx + b
•b is zero
Raoult’s Law
• P soln is the vapor pressure of the solution
• P o solvent solvent is the vapor pressure of the pure
• solvent is the mole fraction of the solvent
Raoult’s Law
•For volatile solutions,
•P total
= (P o
A
)(
A
) + (P o
B
)(
B
)
Raoult’s Law
• is to solutions what the ideal gas law is to gases
• Strong solute-solvent attraction results in a vapor pressure lower than what
Raoult’s Law predicts
6. A carbonated beverage is bottled at 25 ° C with
5.00atm over the CO
2 the liquid. Assuming that the partial pressure of the CO the atmosphere is
2 in in
0.0004atm, calculate the equilibrium concentrations of CO
2 before and after the bottle is opened. The
Henry’s Law constant for
CO
2 is 32Latm/mol at
25 ° C
7. A solution was prepared by adding
20.0g of urea to 125g water at 25 ° C, the temperature at which water’s vapor pressure is 23.76torr. The
observed vapor pressure of the solution was found to be 22.67torr. What is the molar mass of urea?
8. A solution is prepared by mixing 5.81g acetone
(C
3
H
6
O) and 11.9g chloroform (CHCl
35 ° C this solution has a total vapor pressure of
260torr.
3
). At
Is this an ideal solution?
The vapor pressures of pure acetone and chloroform at 35 ° C are
345 and 293torr, respectively.
6.The unopened bottle’s
CO
2 concentration is
0.160 mol/L. The opened bottle’s concentration is
1.2 x 10 -5 mol/L. That’s why it tastes flat.
7. The molar mass of urea is 59.7 g/mol.
8. The expected P
319torr. Since P total total actually 260torr, the solution does not behave ideally.
is is
Colligative Properties
• are dependent upon the
number of solute particles dissolved in solution
–Boiling point
–Freezing point
–Osmotic pressure
Boiling Point
• is the temperature at which a substance’s vapor pressure equals the atmospheric pressure
• What effect does the addition of a nonvolatile solute have on vapor pressure?
Boiling Point
• Thus, the boiling point of a solvent is elevated when a nonvolatile solute is added.
• The amount by which it increases is calculated by…
T b
= k b m solute i
Boiling Point
• T b is the change in boiling point of the solution
• k b is the boiling point constant for the solvent
• m solute solute is the molality of the
• i is the van’t Hoff factor
Boiling Point
•van’t Hoff factor
–the number of particles of dissolved solute per mole of solute…in other words, the number of dissociated particles
Boiling Point
•Know the boiling and point constant for water… k b
= 0.512
°
Ckg/mol
Freezing Point
• is the temperature at which a solid’s vapor pressure equals its liquid’s vapor pressure
• A nonvolatile solute will lower the vapor pressure of the liquid.
Freezing Point
• As the solution is cooled the vapor pressure of the pure solid decreases more rapidly than the vapor pressure of the pure liquid.
• Thus, the freezing point will be lowered.
Freezing Point
T f
= k f m solute i
•Know the freezing point constant for water… k f
= 1.86
°
Ckg/mol
Osmotic Pressure
• is the pressure needed to stop osmosis from occurring
• may be calculated using
= MRTi
Osmotic Presure
• is the osmotic pressure
•M is the molarity of the solution
•R is the gas law constant,
0.08206 Latm/Kmol
•T is the Kelvin temperature
Osmotic Presure
•When pressure greater than is applied, reverse osmosis will occur
–Solute particles are filtered out
–Desalination is an example
•Isotonic solutions
–same osmotic pressure
•Hypertonic solutions
–migration of solvent out of cells results in crenation
•Hypotonic solutions
–migration of solvent into cells results in hemolysis
9. My car’s cooling system contains 2.51kg of water and 2.45kg of ethylene glycol (antifreeze),
C
2
H
6
O
2
. Below what temperature will my engine block freeze?
10. If I were to substitute the ethylene glycol with sodium chloride, below what temperature will my engine block freeze?
11. When 10.0g of camphor are added to 100g of benzene, T o and k
° is 80.1
° C
Ckg/mol, the boiling point of the solution is
81.76
° C. What is the molar mass of the camphor?
12. A 20.0-mg sample of a protein is dissolved in water to make 25.0mL of solution. The osmotic pressure of the solution is 0.56torr at 25 ° C.
What is the molar mass of the protein?
9. -29.2 ° C
10. -62.1 ° C
11. 152g/mol
12. 26550g/mol