Lecture Outline
advertisement

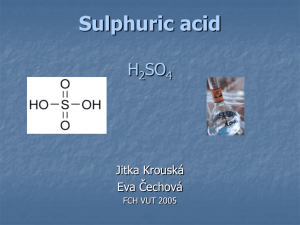
Lecture Outline - Sulfuric Acid - History of Manufacture Development - Manufacture - Oleum Production - Heat Integration Issues / By-products - Markets - Usage in Caprolactam Manufacture History of Manufacture of Sulfuric Acid • One of the oldest industrially applied processes. Discovered by a Persian alchemist in the tenth century. • Saltpeter and sulfur were mixed in a glass container and burned in a moist atmosphere. Acid was collected from the condensed vapors. • In England, 1746, the lead chamber reactor was invented. This invention allowed for higher production rates (<78%). • In England, 1831, a patent was filed that described the oxidation of sulfur dioxide over a platinum catalyst, the Contact Process. This new process increased yields of reaction from 70 to above 95%. • In 1913 BASF was granted a patent for the use of vanadium pentoxide as a catalyst for the Contact Process • By the 1930’s vanadium pentoxide was becoming the dominate catalyst used because of insensitivities to poisons and lower cost. • In 1960 a patent application was filed by Bayer using the so called double-catalyst process (double absorption). World Supply / Demand for Sulfuric Acid (thousands of metric tons, 100% H2SO4) 250,000 200,000 150,000 100,000 50,000 0 Production 2000 1997 1994 1991 1988 1985 Annual Capacity World Production of Sulfuric Acid Canada Mexico Others Japan Latin F-USSR Europe Africa U.S. Asia 0.0 5.0 10.0 15.0 Share (percent) 20.0 25.0 30.0 Manufacture Three Step Process 1) S + O2 2) SO2 + 1/2O2 3) SO3 + H2O SO2 SO3 H2SO4 Oxidation of Sulfur 1) S + O2 SO2 Sulfur Steam 10-12% SO2 Water Air 93% H2SO4 Process: - Air drying tower with acid Primary Generation of SO2 -79% Combustion of Sulfur -9% Recovery from Metallurgic Processes - 5% Regeneration of Spent Acids - Sulfur is injected into burner - Reaction Temperature 2000°F - Exothermic reaction must be cooled - Steam recovered Oxidation of Sulfur Dioxide Contact Process: SO2 Gas -Vanadium pentoxide catalyst Gas Cooling - Exothermic Rxn - Multiple Steps with cooling in between - Double absorption - Heat integration SO3 Gas Oxidation of Sulfur Dioxide Because of the large effect temperature plays on the reaction, multiple catalyst layers had to be used with cooling between each step. Additionally, as the partial pressure of SO3 increases further reaction is limited. This was overcome by removing the SO3 after the third stage to drive the reaction to completion. SO2 Gas SO2 Gas Gas Cooling SO3 Gas SO3 Gas 93% H2SO4 Oxidation of Sulfur Dioxide • Kinetic Effects - Oxidation of sulfur dioxide is slow and reversible - The reaction requires a catalyst and 426.7°C temperatures -The reaction is exothermic and sensitive to excessive heat • Equilibrium Constant (The degree at which the reaction proceeds is temp. dependent) log Kp = 4.956 - 4.678 T T = absolute temp. in kelvin Kp = equilibrium constant as a function of partial pressure of gases Kp = ( PSO3 ) PSO2 PO2 0.5 Oxidation of Sulfur Dioxide Temperature Profile SO2 Gas SO2 Gas Gas Cooling SO3 Gas 510 C 75 C 430 C 200 C SO3 Gas 125 C 93% H2SO4 Oxidation of Sulfur Dioxide Temperature Profile Oxidation of Sulfur Dioxide Typical Catalyst Distribution Catalyst Bed 1 2 3 4 % Catalyst Conversion % 19.4 25.0 26.7 28.9 56 87 99.1 99.7 Overall Production Scheme Oleum Production Sulfuric acid with additional SO3 absorbed 20% Oleum contains 20% SO3 by weight in the oleum Common strengths of oleum are 20, 30, 40, 65 percent. To produce 20 and 30 percent oleum, only requires an additional absorption tower. Oleum is used in reactions where water is excluded SO3 + H2SO4 H2S2O7 (disulfuric acid) Reaction By-products / Heat Integration By-products 57 to 64% of the energy input generates steam Steam energy is used to drive the turbine that supplies power to the main air blower Additional steam remaining is tolled internally for other plant operations SO2/SO3 is vented in small amounts and is federally regulated. Heat Integration Steam is used to pre-heat and vapor from the absorption towers used to cool Minimizes the cost of manufacturing to maximize the profit. Production Considerations Metal corrosion is a big issue in the manufacture of sulfuric acid. Special alloy metals must be used to guard against excessive corrosion. Nickel, chromium, molybdenum, copper, an silicon are the most important elements that enhance corrosion resistance of alloys. Important variables for corrosion Concentration of the acid Temperature of service Speed of flow in pipes and equipment Alloy element make-up Markets for Sulfuric Acid The fertilizer market is the largest U.S. single use for sulfuric acid and consumes 50-65 percent of all produced. Second is the organic chemical industry. Production of plastics and synthetic fibers are examples. Production of TiO2 consumes large quantities of sulfuric acid. TiO2 is a white pigment used in paints and plastics. In the metal industry sulfuric acid is used for pickling ferrous and nonferrous materials and in the recovery of copper, nickel, and zinc from low-grade ores. Finally, the petroleum industry uses acid as a catalyst for various reactions. Acid Strengths Associated End Uses Percent H2SO4 35.67 Uses Storage batteries, electric utilities 62.18 Normal superphosphate and other fertilizers 69.65 77.67 Normal superphosphate and other fertilizers, isopropyl and sec-butyl alcohols 80.00 Copper leaching 93.19 Phosphoric acid, tianium dioxide, steel pickling, regenerating ion exchange resins of utilities 98-99 Chlorine drying, alkylation, boric acid 104.50 Surfactants, nitrations, hydrofluoric acid 106.75 109.00 Explosives 111.25 113.50 114.63 Reagent manufacture, organic sulfonations, blending with weaker acids Usage in Caprolactam Manufacture Production and consumption figures for caprolactam manufacture Caprolactam Production Rate 120,000 ton/yr H2SO4 Consumption (100% Acid) 636 kg per ton of CPL Oleum Consumption 1300kg per ton of CPL Ammonium Sulfate Production 312,000 ton/yr