CHEM 1110 Fall 2001 - Test 4 for Dr. Condon's
advertisement

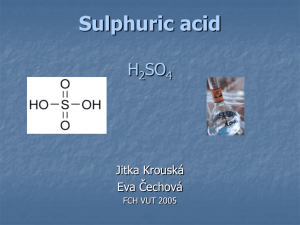
CHE M 11 10 Fall 20 01 - Test 4 Page 1 CHEM 1110 Fall 2001 - Test 4 for Dr. Condon’s sections 1. Which of the following is a redox reaction? a. LiH + H2O ! LiOH + H2 b. AgNO3(aq) + NaCl(aq) ! AgCl(s) + NaNO3(aq) c. HCl + KOH ! KCl + H2O d. AgCl(s) + 2NH3(aq) ! Ag(NH3)2Cl(aq) e. CaO(s) + CO2(g) ! CaCO3(s) In the following, questions 2 through 6, x is the stoichiometric coefficient that is not provided. 2. What is(are) the expected product(s) from the reaction: Mg + xS8 ! ? a. MgS8 b. Mg2S c. MgS2 + Mg6S d. Mg3S e. MgS 3. What is(are) the expected product(s) from the reaction: P4 + xF2 ! ? a. PF2 and PF6 b. P2F c. PF3 and PF5 d. P4F2 e. FP and FP7 4. What products are obtained when sulfur reacts with oxygen? S8 + xO2 ! ? a. SO and SO3 b. SO2 and SO3 c. SO2 and SO6 d. SO2 and S2O e. SO and SO2 5. What product(s) is(are) obtained when nitrogen reacts with hydrogen? N2 + xH2 ! ? a. NH3 and NH5 b. NH5 c. N2H2 d. HN3 e. NH3 CHE M 11 10 Fall 20 01 - Test 4 Page 2 6. What product(s) is(are) obtained when calcium hydride reacts with water? CaH2 + xH2O ! ? a. CaH4O2 b. CaH3O c. CaO + H2O2 d. CaOH + H2O2 e. Ca(OH)2 + H2 7. Which of the following is the Lewis dot structure for ethene (H2CCH2)? a. b. d. e. c. 8. Which of the following has at least one lone (unshared, non-bonding) pair of electrons about the central atom? a. SO3 b. SF6 c. HCCH d. CH2Cl2 e. IF2! d. H2SO4 e. HCN 9. Which of the following has at least one triple bond? a. SO2 b. CO2 c. O2 (For H2SO4 use the sequence: (HO)2SO2, i.e. the Hs are bonded to Os and all four O are bonded to S.) 10. Which of the following has a resonance structure? a. CO32! b. CH4 c. H2CCH2 d. H2SO4 e. SF5 11. Which of the following has more than 8 electrons about the central atom? a. HCN b. I3! c. NH3 d. H2SO4 e. CH2F2 CHE M 11 10 Fall 20 01 - Test 4 Page 3 12. Which of the following has a dipole moment? a. I3! b. CH2F2 c. H2CCH2 d. SO3 e. SF6 13. Which of the following has the highest London forces with itself? a. Ne b. Ar c. Kr d. Xe e. Rn 14. Which of the following molecules can hydrogen bond with molecules that are the same? a. CH3COOH b. H3COCH3 c. (CH3)3N d. CH4 e. PH3 15. Which of the following forces is the strongest? a. London forces b. hydrogen bonding c. ionic-ionic attractions d. dipole-dipole attractions e. they are all about the same 16. Which of the following redox reactions with occur spontaneously? a. Zn2+ + Cu(s) ! Cu2+ + Zn(s) b. Ni2+ + Cu(s) ! Cu2+ + Ni(s) c. 2I! + ClO! + H2O ! I2 + Cl! + 2OH! d. Cu(s) + 2H+ ! Cu2+ + H2(g) e. 2Na+ + 2Cl! ! 2Na(s) + Cl2 17. Based upon the strength of intermolecular forces, which of the following would have the highest boiling point? a. H2O b. NH3 c. H2Se d. H2Te e. H2S CHE M 11 10 Fall 20 01 - Test 4 Page 4 18. What is the geometry for the molecule SF6? a. tetrahedral b. trigonal pyramid c. bent 120° d. octahedral e. bent 108° 19. What is the hybrid for carbon in the molecule HCCH? a. sp b. sp2 c. sp3 d. sp3d e. sp3d2 20. In which of the following molecules is there hindered rotation? a. CH2F2 b. H3CCF3 c. H3CCH3 d. H2CCH2 e. HCCH CHE M 11 10 Fall 20 01 - Test 4 1.a 2.e 3.c 4.b 5.e 6.e 7.d 8.e 9.e 10.a 11.b 12.b 13.e 14.a 15.c 16.c 17.a 18.d 19.a 20.d Page 5