Pigment Notes
advertisement

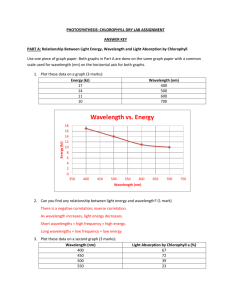
Electromagnetic energy › Solar energy or radiation which travels in space a rhythmic waves and can be measured in photons Wavelength › The distance between crests of adjacent waves such as those of the electromagnetic spectrum Visible Light › The radiation our eyes see as different colors Fig. 10-6 10–5 nm 10–3 nm 103 nm 1 nm Gamma X-rays rays UV 106 nm Infrared 1m (109 nm) 103 m Microwaves Radio waves Visible light 380 450 500 Shorter wavelength Higher energy 550 600 650 700 750 nm Longer wavelength Lower energy Pigments › Substances that absorb some wavelengths of light and reflect others › Different pigments absorb/reflect different wavelengths Photon › A fixed quantity of light energy › The shorter the wavelength of light the greater the energy of a photon Fig. 10-7 Light Reflected light Chloroplast Absorbed light Granum Transmitted light Which wavelengths are absorbed most? › Primarily blue and red Why do plants appear green? › They reflect green light What is the role of accessory pigments? › They allow the plant to absorb energy from more the visible light spectrum – making the plant more efficient. Fig. 10-9 RESULTS Chlorophyll a Chlorophyll b Carotenoids (a) Absorption spectra 400 500 600 700 Wavelength of light (nm) (b) Action spectrum Aerobic bacteria Filament of alga (c) Engelmann’s experiment 400 500 600 700 When a pigment absorbs light, it goes from a ground state to an excited state, which is unstable When excited electrons fall back to the ground state, photons are given off, an afterglow called fluorescence If illuminated, an isolated solution of chlorophyll will fluoresce, giving off light and heat Photosystem (1st part) › Light harvesting complexes pigment molecules bounded by proteins; absorb transfer energy to chlorophyll a of reaction center Photosystem (2nd part) › Reaction Center Chlorophyll a and a primary electron acceptor Chlorophyll a uses energy from light harvesting complexes to pass a pair of electrons to the primary electron acceptor