File
advertisement

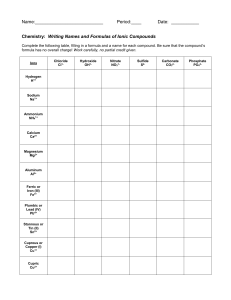
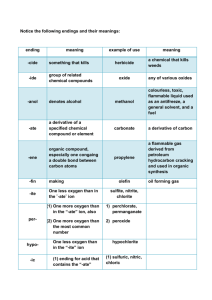
NOMENCLATURE Nomenclature can be defined as the terminology of chemical compounds. It represents the basic "language of chemistry" and, just as the student who is studying French or Spanish must learn the terminology of those languages, so must the chemistry student learn the basic terminology of the discipline. Life certainly becomes a bit easier, and safer for everyone around, if chemistry students are able to read the formulas on the bottles of stock solutions correctly when they have only the names of these substances on their lab sheet or vice versa. Okay, so it's a given......one of the basic, most important tasks that a chemistry student needs to master early on is that of Naming Compounds and Writing Formulas. There are several things that a student can do to make these tasks less tedious. Learn the correct symbols for the elements. (This is similar to reciting the alphabet...a, b, c..., as well as identifying the symbols we use for each letter.) Learn the names and formulas of seven (7) acids and ammonia. The acids and their formulas, along with ammonia and its formula are: hydrochloric acid HCl nitric acid HNO3 acetic acid HC2H3O2 perchloric acid HClO4 carbonic acid H2CO3 sulfuric acid H2SO4 phosphoric acid H3PO4 ammonia NH3 Learn the meaning of several important prefixes and suffixes as they relate to the formulas of polyatomic ions, cation charges, and anions. Prefix/Sufix Meaning Example(s) -ate denotes the most common number of oxygen atoms -ite one less oxygen than in the "-ate" ion per- (1) one more oxygen than in the "-ate" ion, also sulfate, nitrate, chlorate sulfite, nitrite, chlorite (2) one more oxygen than the most common number (1) perchlorate, permanganate (2) peroxide hypo- one less oxygen than in the "-ite" ion hypochlorite ide- ion contains only the atoms whose names are heard hydroxide, chloride, sulfide nitride -ic (1) ending for acid that contains the "-ate" form of the ion (2) indicates the higher oxidation number of some cations (older method, but still used) (1) sulfuric, nitric, chloric (2) ferric is iron in the 3+ state, cupric is copper in the 2+ state -ous (1) ending for acid that contains the "-ite" form of the ion (2) indicates the lower oxidation number of some cations (also older method, but still used) sulfurous, nitrous, chlorous (1) (2) ferrous is iron in the 2+ state, cuprous is copper in the 1+ state hydro- -ic hydrosulfuric prefix and suffix used with acids whose anions contain no oxygen hydrochloric, Notice that the acids suggested for memorization, other than hydrochloric, all end in -ic, and thus contain the -ate form of the polyatomic ion. You can therefore, quickly identify these ions when you see them in the formula for some other compound or easily write the formula for a compound that contains one of these ions. You can then use the meanings for the other prefixes and suffixes to expand your repetoire of ions without having to memorize each of them separately. Learn the names of the prefixes that are used to denote one (1) through ten (10) atoms in a formula. 1 = mono- 2 = di- 3 = tri- 4 = tetra- 5 = penta- 6 = hexa- 7 = hepta- 9 = nona- 8 = octa- 10 = deca- Applying These Rules There are two kinds of compounds for which students in high school and general chemistry are usually expected to write formulas or to name. They are ionic compounds and binary covalent compounds. A third kind that are also helpful to learn are those of the coordination compounds, but they have their own set of rules for naming or writing and will not be covered here. Writing Formulas (Notes from: http://www.files.chem.vt.edu/RVGS/ACT/notes/Nomenclature.html) Covalent Compounds: Nm – Nm (number name it) Eg. CO2 It’s an Nm-Nm (look at periodic table) so: Carbon dioxide Never number name first element in the compound on number name the ones that follow the first Ionic compounds : M-Nm ( just name it) Eg. NaCl Its M-Nm ( look at periodic table) so : Sodium chloride