Chemistry: Names and Formulas of Compounds
advertisement
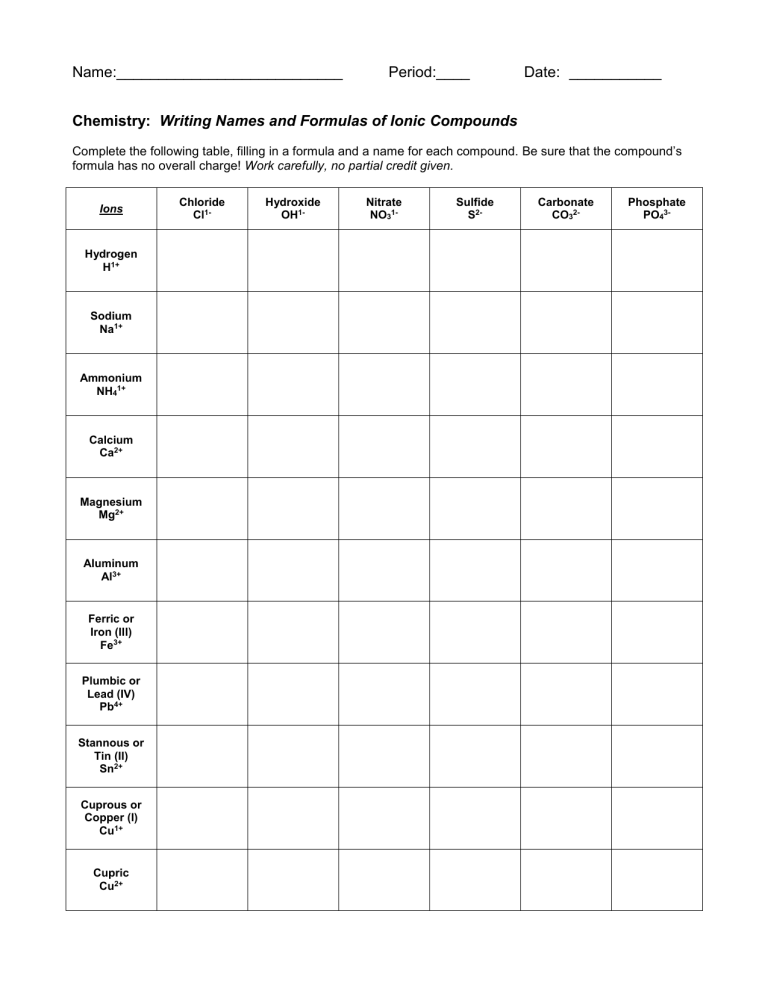
Name:___________________________ Period:____ Date: ___________ Chemistry: Writing Names and Formulas of Ionic Compounds Complete the following table, filling in a formula and a name for each compound. Be sure that the compound’s formula has no overall charge! Work carefully, no partial credit given. Ions Hydrogen H1+ Sodium Na1+ Ammonium NH41+ Calcium Ca2+ Magnesium Mg2+ Aluminum Al3+ Ferric or Iron (III) Fe3+ Plumbic or Lead (IV) Pb4+ Stannous or Tin (II) Sn2+ Cuprous or Copper (I) Cu1+ Cupric Cu2+ Chloride Cl1- Hydroxide OH1- Nitrate NO31- Sulfide S2- Carbonate CO32- Phosphate PO43- Name:___________________________ Period:____ Date: ___________ Chemistry: Names and Formulas of Compounds Write either the traditional or Stock System name for each of the following compounds. 1. Na2S 1. ______________________ 2. NH4Cl 2. ______________________ 3. CuF 3. ______________________ 4. CuF2 4. ______________________ 5. PbSO4 5. ______________________ 6. CO 6. ______________________ 7. Al2O3 7. ______________________ 8. N2O4 8. ______________________ 9. Br2 9. ______________________ 10. P2O5 10. ______________________ Write the chemical formula for each of the following compounds. 11. tin (II) chloride 11. ______________________ 12. cuprous nitrate 12. ______________________ 13. ammonium sulfate 13. ______________________ 14. magnesium nitride 14. ______________________ 15. nitrogen monoxide 15. ______________________ 16. sulfur dioxide 16. ______________________ 17. iron (II) oxide 17. ______________________ 18. diphosphorus pentoxide 18. ______________________ 19. calcium phosphate 19. ______________________ 20. calcium phophide 20. ______________________ Name:___________________________ Period:____ Date: ___________ Chemistry: Errors in Chemical Formulas and Nomenclature Each of the following formulas or chemical names contains an error. Correct each example. 1. aluminum (III) iodide 1. ______________________ 2. dihydrogen oxide 2. ______________________ 3. ferric (III) nitride 3. ______________________ 4. tin (III) nitrate 4. ______________________ 5. leadic sulfide 5. ______________________ Chemistry: Acid Nomenclature Name each of the following acids. Remember that acid formulas begin with H and that comparing the number of oxygens in an acid’s formula to the number of oxygens in the “-ate” polyatomic anion is very helpful! (chlorate = ClO31-) 1. HCl 1. ______________________ 2. HClO3 2. ______________________ 3. HClO2 3. ______________________ 4. HClO 4. ______________________ 5. HClO4 5. ______________________ Write the formula for each of the following acids. Remember that acid formulas begin with H and comparing the number of oxygens in an acid’s formula to the number of oxygens in the “-ate” polyatomic anion is very helpful! (bromate = BrO31-) 6. hydrobromic acid 6. ______________________ 7. bromic acid 7. ______________________ 8. bromous acid 8. ______________________ 9. hypobromous acid 9. ______________________ 10. perbromic acid 10. ______________________ Name:___________________________ Period:____ Date: ___________ Chemistry: Nomenclature Flowchart Use the space below to copy the naming flowchart form your textbook, or create an original one of your own that uses the Type I, Type II, Type III and Type IV groupings from the text.