Mass and the Mole
advertisement

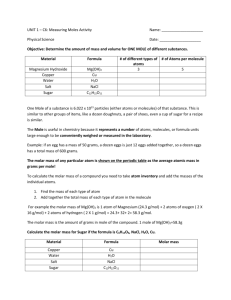
Notebook Setup Assignment Mass and the Mole CORNELL notes Moles to Mass Example & Mass to Moles Example Mass to Atoms & Atoms to Mass Examples Calculations Involving the Molar Mass of an Element handout Page Number 51 52 53 54 Mass and the Mole Learning Objectives Relate the mass of an atom to the mass of a mole of atoms. Convert between number of moles and the mass of an element. Convert between number of moles and number of atoms of an element. Introduction • Do eggs come in different sizes? • Does the size of the eggs affect how many you get if you buy a dozen eggs? • A dozen always contains the SAME number of eggs but a dozen eggs will have different masses depending on the size of the eggs. Introduction • Does a dozen limes have the same mass as a dozen eggs? • Because eggs and limes differ in size and composition, they have different masses. NOTES START HERE • For the same reason, one-mole quantities of two different substances have different masses. – Example: one mole of carbon and one mole of copper have the SAME number of particles but different masses Molar Mass • Molar mass is the mass in grams of one mole of any pure substance. • The molar mass of any element is numerically equal to its atomic mass and has the units grams/mol. • The mass of an atom is also equal to the mass in grams of one mole of the element. Conversions Involving Mass Real World Problem • Imagine that our class bought jelly beans in bulk to sell by the dozen at a candy sale. We soon realize that it is too much work to count out each dozen, so we decide to measure the jelly beans by mass. We find that the mass of 1 dozen jelly beans is 35 g. • What mass of jelly beans should you measure if a customer wants 5 dozen jelly beans? Problem Setup 1. Write the given and needed units. 2. Write a unit plan to convert the given unit to the needed unit. 3. Write conversion factors that connect the units. 4. Set up the problem. Use conversion factors to cancel the given unit and provide the needed unit. 5. Calculate the answer. Multiply numbers that are across from one another and divide numbers that are underneath. Real World Problem Step 1: known 5 dozen unknown # of jelly beans 1 dozen = 12 roses Step 2: dozen # of jelly beans Step 3: 1 dozen = 12 jelly beans 1 𝑑𝑜𝑧𝑒𝑛 12 𝑗𝑒𝑙𝑙𝑦 𝑏𝑒𝑎𝑛𝑠 Step 4: Step 5: 5 dozen or 12 𝑗𝑒𝑙𝑙𝑦 𝑏𝑒𝑎𝑛𝑠 1 𝑑𝑜𝑧𝑒𝑛 12 jelly beans 1 dozen 60 jelly beans Moles to Mass Example Calculate the mass in grams of 0.0450 mol chromium (Cr). Mass to Moles Example How many moles of calcium (Ca) are in 525 g Ca? Mass to Atoms Example How many atoms of gold are in a U.S. Eagle, a gold alloy bullion coin with a mass of 31.1 g Au? Atoms to Mass Example A party balloon contains 5.50 x 1022 atoms of helium gas. What is the mass, in grams, of the helium? Summary • The mass in grams of one more of any pure substance is called its molar mass. • The molar mass of an element is numerically equivalent to its atomic mass. • The molar mass of any substance is the mass in grams of Avogadro’s number of representative particles of the substance. • Molar mass is used to convert from moles to mass. The inverse of molar mass is used to convert from mass to moles.