Chemistry vocab 3
advertisement
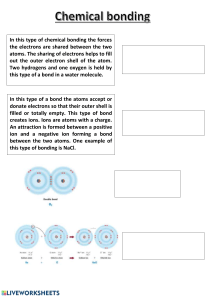
Chemistry Vocabulary Part 3 Meghan Goodell Chemical Equation Shorthand form, used for writing what reactants are used and what products are formed in a chemical reaction Subscript The term written beneath an element that indicates the number of atoms of this element or compound. Chemical Formula A way of expressing information about the number of atoms that make up particular chemical compounds. Chemical Bond An electrical force linking atoms ion an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving the atom a net positive or negative electrical charge. Cation a positively charged ion Anion Negatively charged ion Metallic bond Bond formed when metal atoms share their pooled electrons Ionic bond Attraction that holds oppositely charged ions close together Covalent bond Chemical bond formed when atoms share electrons Octet Rule Atoms with 8 electrons on their outer energy level (shell) are stable (unreactive) Valence Electrons An electron in an outer shell(energy level) of an atom that can participate in forming chemical bonds with other atoms.