Chapter 5
advertisement

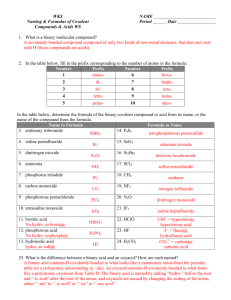
Nomenclature 5.1 Naming Compounds Binary compounds: 2 elements are joined by a bond Ionic compound Metal + nonmetal Covalent compound Nonmetal + nonmetal 5.2 Naming Binary Compounds Type I Metals present forms only type of cation E.g +1 charge Type II Metals can form two or more cations that have different charges E.g +2, +3 etc… charges Rules for Naming Type I The cation is always named first and the anion second The name of cation stays the same Na+ = Sodium The name of anion takes the first part of the root name and adding –ide Cl- = Cloride Steps in naming a compound CsF Step 1 Identify the cations and anion (as well as the group number Step 2 Name the cation Step 3 Name the anion Step 4 Name the compound by combining the names Common Type I Ions Example Name each binary compound AlCl3 ZnS BaH2 MgO Naming Binary Ionic Compound Type II Contains metals mostly from the transitional group Must use Roman numeral to indicate their charges E.g +2 = II +3 = III +4 = IV Common Type II Ions Example Give the system of each of the following compounds PbCl4 CoCl3 Fe2O3 CrI3 5.3 Naming Binary Contain Only Nonmetals (Type III) The first element in the formula is named first and the full element name is used The second element is named as though it were an anion ( ide ending) Prefixes is used to indicate the number of atoms present. (prefer to table 5.3) The prefix mono- is never used for naming the first element. Example Name the following compound CCl4 SiO2 P4O6 NO Naming Binary Compounds: a Review 5.5 Naming Compounds that Contain Polyatomic Ions Polyatomic ions – two or more nonmetals are covalently bonded and have charges Can be positively charge or negatively charge Oxyanion – nonmetal is bonded to an oxygen element and carried a negative charge Common Polyatomic Ions Example Name the following compounds Na2CO3 CsClO3 Fe(OH)2 Ca(HCO3)2 Naming Acids Acids – substance that produces H+ when dissolve in water Prefix hydro is used when the anion does not contain oxygen and the suffix –ic is attached to the root name of element When the anion contains oxygen, the acid name is formed from the root name of the central element of the anion or the anion name, with a suffix of –ic or -ous Flow Chart Example Name the following acids HCl HF HBrO4 H2SO4 HCN