Signs of a chemical change
advertisement

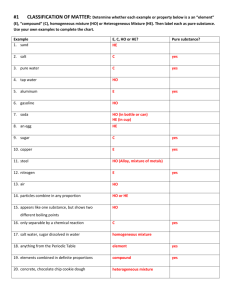
Name: ______________________ Class: ______ Grade 7 Module 2 Chemical properties and Chemical Reactions Mark Module checked Date: ____ 10 Comment: Miss Liza Dippenaar Date: _________________ Objective: _____________________________________________ _____________________________________________________ ------------------------------------------------------------------------------------------------------------ Checking prior knowledge: Physical or Chemical Change? Indicate with a ‘P’ or a ‘C’ which type of change is taking place. 1. ____________ glass breaking 2. ____________ hammering wood together 3. ____________ a rusting bicycle 4. ____________ melting butter 5. ____________ separate sand from gravel 6. ____________ bleaching your hair 7. ____________ frying an egg 8. ____________ squeeze oranges for juice 9. ____________ melting ice 10. ____________ mixing salt and water 11. ____________ mixing oil and water 12. ____________ water evaporating 13. ____________ cutting grass 14. ____________ burning leaves 15. ____________ fireworks exploding 16. ____________ cutting your hair 17. ____________ crushing a can 18. ____________ boiling water Fill in the Blanks: ____________ properties can be observed without chemically changing matter. ____________ properties describe how a substance interacts with other substances. ____________ have definite shapes and definite volumes. ____________ have indefinite shapes and definite volumes. ____________ have indefinite shapes and indefinite volumes. Phase changes are ____________ changes. ____________ point is the temperature at which a liquid turns to a solid. It is also equal to the ____________ point which is the temperature at which a ____________ turns to a ____________. ____________ point is the temperature at which a liquid turns to a gas, and ____________ point is the temperature at which a gas turns to a liquid. Occasionally, a solid turns directly into a gas without turning into a liquid first. This is called ____________. Label these properties as chemical (C) or physical (P). Be certain to know the definition of each of these properties. combustibility _____ malleability _____ weight _____ failure to react _____ ductility _____ texture _____ density _____ tendency to corrode _____ volume _____ melting point _____ odor _____ flammability _____ Signs of a chemical change: 1) Change in color 2) Change in odor 3) Production of heat 4) Fizzing and foaming 5) Sound given off 6) Light given of Assessment – Chemical changes Date: ___________________ ------------------------------------------------------------------------- 1) In your own words, what is a chemical change? ________________________________________________ ________________________________________________ 2) Give 2 examples of a chemical change. ________________________________________________ ________________________________________________ 3) For each of the following, state whether it is a chemical change or a physical change. Physical change/chemical change Physical change/chemical change Physical change/chemical change Physical change/chemical change Mini- Class Project Your task is to describe the properties and purposes of some common objects. Some of the properties used to describe common materials such as food, clothing, writing materials and sports equipment are strong, hard, fluffy, solid, smooth, soft, liquid, bends, floats, shiny, sinks, translucent, opaque, flexible, sticky, light, runny or heavy. Here’s an example of a school cap. Purpose (what it has to do) Protect your face from the sun Change size to fit your head size Materials Cotton Properties Flexible Plastic Light Velcro Cool Soft In each box below, draw a common object and then describe its purposes, materials and properties.