Nucleotide metabolism
advertisement

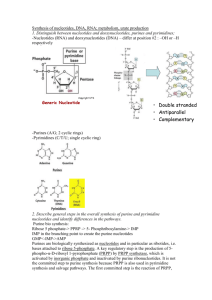
Nucleotide metabolism “GOUT, n. A physician's name for the rheumatism of a rich patient.” THE DEVIL’S DICTIONARY. AMBROSE BIERCE 1 BIOSYNTHESIS AND DEGRADATION OF NUCLEOTIDES • Nucleotides are composed of a nitrogenous base, sugar and phosphate (s) base and sugar only = nucleoside • Nucleotides are precursors of nucleic acids (DNA, RNA), energy rich compounds (ATP, GTP, UTP, CTP), coenzymes (NAD/P+ , FAD, CoA, SAM, Vit B 12) and signaling molecules (cAMP, cGMP) • Small amounts of nucleic acids in the diet • Pancreatic ribonuclease and deoxyribonuclease change the nucleic acids into their nucleotide monomers • Intestinal phosphatase converts nucleotides into nucleosides • The greater portion of nucleosides is modified and excreted; a small amount is recycled back to nucleotides 2 • The bases of nucleotides are either pyrimidine or purine • Pyrimidines are characterized by a six-membered heterocyclic aromatic ring with two nitrogens • Purines have an additional five-membered imidazole ring fused with the aromatic ring of pyrimidines • The common naturally occurring pyrimidines are cytosine, uracil and thymine (5-methyuracil) • Purines: adenine and guanine • Other naturally occurring purine derivatives include xanthine, hypoxanthine and uric acid 3 4 • The sugar found in ribonucleotides is D-ribose and in deoxyribonucleotides is 2-deoxy D-ribose • The pentose sugars are attached to the nitrogenous bases through a β-N-glycosidic bond to give nucleosides 5 • In pentoses of nucleosides (and nucleotides), the carbon , numbers are given a prime designation ( ) to distinguish them from the numbered atoms of the bases • Nucleosides are named by adding –idine to the root name of pyrimidines and –osine to the root name of purines: cytidine, thymidine, uridine, adenosine, guanosine, inosine • The prefix deoxy- added in the case of deoxyribonucleosides Deoxythymidine, deoxyuridine, … 6 • Nucleosides are converted to nucleotides through the linking of a phosphate with the 5’ hydroxyl group of ribose or deoxyribose Adenosine 5’-monophosphate (AMP), cytidine 5’ monophosphate (CMP),… • More phosphates could be added to give nucleoside di- and triphosphates ; or a single phosphate group can be esterified with two hydroxyls of the pentose to give a cyclic nucleotide 7 • Two types of pathways lead to the synthesis of nucleotides: the de novo pathways and the salvage pathways • de novo synthesis begins with amino acids, ribose 5phosphate and CO2 • Salvage pathways recycle free bases and nucleosides obtained from nucleic acid breakdown (from nucleic acid turnover or from the diet) • During de novo synthesis new bases are synthesized purine nucleotides are constructed a few atoms at a time on a ribose-based structure the framework for pyrimidine bases is first synthesized and is then attached to ribose and modified further • On the other hand, free bases can serve as intermediates in salvage pathways 8 Purine nucleotide synthesis De novo: is initiated using an activated form of ribose Ribose-5-phosphate from PPP reacts with ATP to produce 5-phospho-α-D-ribosyl-1-pyrophosphate (PRPP) • The pyrophosphate of PRPP is displaced by the amide nitrogen of glutamine in a reaction catalyzed by glutamine PRPP amidotransferase The committed step of de novo purine synthesis 9 • Once 5-phospho-β-D-ribosylamine is formed, the building of the purine ring begins First, the whole of a glycine molecule is added to 5-phosphoβ-D-ribosylamine and phosphoribosylglycinamide is formed Additional nitrogen comes from glutamine and aspartate N10-formyl THF and CO2 provide additional carbons Four ATP molecules are used in the process After eight steps, the first purine nucleotide inosine monophosphate (IMP) is produced The sources of the atoms in the purine ring 10 • IMP is converted to AMP or GMP: the keto group of IMP is replaced by an amino group (provided by aspartate) in AMP; requires GTP the dehydrogenation of IMP using NAD+ yields xanthosine monophosphate (XMP); XMP is then converted to GMP through the donation of the amino group of glutamine; requires ATP 11 12 • Nucleoside triphosphates are the most common types of nucleotide in metabolism • The production of nucleoside diphosphates requires specific (for type of base) nucleoside monophosphate kinases whereas nucleoside triphosphate formation is catalyzed by nucleoside diphosphate kinases that are not specific for sugar or base The regulation of de novo purine synthesis • The committed step is inhibited by excess levels of IMP, GMP and AMP • An excess of GMP inhibits the formation of XMP whereas an excess of AMP inhibits the synthesis of adenylosuccinate • ATP is needed for the synthesis of GMP and GTP is required for the synthesis of AMP the pools of the two nucleotides are balanced 13 14 • PRPP synthesis is allostericaly inhibited by ADP, GDP and other metabolites from other pathways which use PRPP as a precursor o Because the de novo pathway consumes much energy, cells synthesize a significant portion of their nucleotides by salvage pathways Purine salvage pathways • Free purine bases or (deoxy) ribonucleosides can be changed back to mono nucleotides • The liver is the major site of purine nucleotide metabolism; it provides purines and purine nucleosides for other tissues in some tissues, the de novo pathway produces little or no purine nucleotides – these tissues are dependent upon export from the liver 15 • In most tissues AMP is changed by 5’-nucleotidase to adenosine • Adenosine can then be deaminated by adenosine deaminase to inosine and inosine can then be converted to IMP • In adenosine deaminase deficiency large concentrations of dATP (because adenosine deaminase acts also on deoxyadenosine) inhibit ribonucleotide reductase; consequently DNA synthesis is depressed Severe combined immunodeficiency (SCID) - T and B lymphocytes are mainly affected • Adenosine, unlike the other purine nucleosides, can be converted to AMP by adenosine kinase • Muscular work is accompanied by the production of ammonia, the immediate source of which is AMP • AMP is changed to IMP with the associated release of NH4+; the enzyme involved is known as AMP aminohydrolase or AMP 16 deaminase • IMP is changed back to AMP through the de novo pathway • The net effect is the generation of fumarate which can serve an anaplerotic role This is known as the purine nucleotide cycle It operates in the brain as well as in the muscles • Adenosine is thought to be a local hormone or autocoid During strenuous activity, muscles release adenosine which has a vasodilatory effect leading to increased delivery of oxygen and nutrients Adenosine slows the heart rate by blocking the flow of electrical current • Purine nucleoside phosphorylase converts inosine, guanosine and xanthosine to hypoxanthine, guanine and xanthine, respectively • Deficiency of purine nucleoside phosphorylase affects T cells17 • Purine bases can react with PRPP under the influence of phosphoribosyltransferases • Adenosine phosphoribosyl transferase catalyzes the reaction of adenine with PRPP to give AMP • Hypoxanthine-guanine phosphoribosyl transferase (HGPRT) catalyses the reaction of guanine and hypoxanthine with PRPP • The deficiency of HGPRT causes the Lesch-Nyhan syndrome guanine and hypoxanthine will be degraded instead of being salvaged mental retardation, self-mutilation 18 19 Purine catabolism • GMP catabolism is initiated by a 5'-nucleotidase, which converts GMP to guanosine • The release of ribose from guanosine is catalyzed by purine nucleoside phosphorylase • Guanine is then deaminated to xanthine • There are two alternative pathways for catabolism of AMP • The major route of AMP catabolism involves generation of IMP by AMP deaminase, followed by removal of the phosphate group by 5'-nucleotidase • In the alternative pathway of AMP catabolism, 5'-nucleotidase converts AMP to adenosine, which is then deaminated by adenosine deaminase • Purine nucleoside phosphorylase then catalyzes release of the ribose moiety from inosine produced in both ways 20 • The final and common enzyme in the pathway of purine catabolism is xanthine oxidase, a molybdenumcontaining flavoenzyme that uses molecular oxygen and produces hydrogen peroxide • Xanthine oxidase oxidizes both hypoxanthine (to xanthine) and xanthine (to uric acid) 21 • In some plants xanthine is trimethylated to caffeine During periods of extended wakefulness, metabolic activities in the brain lead to the accumulation of adenosine Adenosine induces sleepiness; caffeine promotes wakefulness by blocking the interaction of extracellular adenosine with neuronal receptors • In primates uric acid is excreted in the urine as it is • In other mammals urate oxidase changes uric acid to allantoin; in other organisms allantoin to allantoate to urea to NH4+ • Urate is present in human serum at levels near precipitation • This may be because of the use of urate as an anti-oxidant • Gout (gutta, in Latin “drop”) is a common disorder resulting from hyperuricemia: > 7 mg/dL in males, > 6 mg/dL in females • It causes inflammation of the joints– gouty arthritis 22 Crystals of sodium urate are deposited in the joints The deposition attracts macrophages that engulf the crystals Lysosomal membranes of the macrophages are ruptured by the crystals; this results in the release of lysosomal enzymes into the tissues Histamine is also released from mast cells Visible structures known as tophi may form near joints causing deformities • The deposition of urate crystals within the renal tubules causes impaired renal function • Primary gout results from genetic defects in purine metabolism A n overactive PRPP synthase leading to an increase in purine synthesis Deficiency of HGPRT means less salvage reactions • Glucose-6-phosphatase deficiency leads to the accumulation of glucose-6-phosphate which will be diverted to ribose-5-phosphate 23 and then to PRPP • Secondary or acquired gout is caused by various factors that can cause an overproduction of urate or its underexcretion by the kidneys • Gout can be treated with dietary control and drugs • Allopurinol is a suicide inhibitor of xanthine oxidase Allopurinol is changed to alloxanthine (oxypurinol) which tightly binds and inactivates xanthine oxidase Hypoxanthine and xanthine can be excreted in the urine • Allopurinol can also react with PRPP producing allopurinol ribonucleotide thereby reducing purine synthesis • Colchicine inhibits the polymerization of tubulin into microtubules The division and movement of and phagocytosis by white blood cells is inhibited Lekotriene B4 and histamine release is curtailed • In earlier times gout was thought to be associated with rich diets 24 and excessive consumption of alcoholic drinks • Lead salts used to be added as preservatives in alcoholic drinks; pots lined with lead-containing materials • Lead inhibits guanine aminohydrolase leading to the accumulation of guanine in the joints – Saturnine gout • The bone stores Pb+2 because it is divalent just like Ca+2; through time Pb+2 is transported to other tissues including the kidney • Treatment by chelating agents like ethylenediaminetetraaceticacid (EDTA) 25 • • • • • • The de novo biosynthesis of pyrimidine nucleotides The synthesis of pyrimidines begins with the formation of carbamoyl phosphate by CPS II – a cytosolic isozyme Uses glutamine as nitrogen donor instead of free ammonia Carbamoyl phosphate reacts with aspartate to give carbamoyl aspartate; the enzyme is aspartate transcarbamoylase (ATCase) The closure of the pyrimidine ring is then catalyzed by dehydration through the action of dihydroorotase Dihyrdroorotate is then oxidized to orotate by dihydroorotate dehydrogenase ( a flavoprotein) the NADH produced can enter the electron transport chain CPS II, aspartate transcarbamoylase and dihydroorotate dehydrogenase are part of a single trifunctional protein known as CAD At this point, the basic framework of pyrimidine bases has been 26 constructed 27 • Orotate and PRPP react under the catalysis of orotate phosphoribosyl transferase to yield orotidine-5-phosphate (OMP) • OMP is then decarboxylated to uridylate (UMP) by OMP decarboxylase UMP is phosphorylated to UTP UTP receives an amide group from glutamine to become cytidine triphosphate (CTP) • Both orotate phosphoribosyl transferase and OMP decarboxylase activities are located on a protein called UMP synthase The deficiency of UMP synthase leads to a condition as orotic aciduria - orotate in the urine, retarded growth, anemia Can be treated with uridine which inhibits the production of orotate and provides building blocks for nucleic acid 28 synthesis The sources of the atoms in the pyrimidine ring 29 • • • • • The regulation of de novo pyrimidine synthesis CPS II is activated by PRPP and inhibited by UTP As cells approach the S-phase of the cell cycle CPS II becomes more sensitive to activation by PRPP and less sensitive to inhibition by UTP phosphorylation of CPS II by MAPK at a specific site leads to an easily activated enzyme while phosphorylation at another site would lead to an easily inhibited enzyme The ATCase reaction is the committed step of pyrimidine synthesis inhibited by CTP and activated by ATP in bacteria The synthesis of thymidylate DNA contains thymine instead or uracil The route to thymidine is through deoxyribonucleotides 30 • Deoxyribonucelotides are synthesized from the ribonucleotides by reduction of the 2’-carbon of D-ribose The enzyme involved is ribonucleotide reductase The substrates are diphosphonucleotides The reducing equivalents are ultimately donated by NADPH via thioredoxin or glutaredoxin • UDP is changed to dUDP; dUDP is phosphorylated to dUTP and then pyrophosphate is removed from dUTP to produce dUMP • Or dCDP can be changed to dCMP which is deaminated to dUMP • dUMP receives a methyl group from N5,N10- methylene THF to give dTMP which will be phosphorylated to dTTP 31 the enzyme is thymidylate synthase 32 33 34 • The thymidylate synthase reaction converts THF to dihydrofolate (DHF) • THF is regenerated through the action of dihydrofolate reductase with NADPH providing the reducing equivalents • Rapidly dividing cells require ample supplies of thymidylate for DNA synthesis; nucleotide biosynthesis can be targeted to design important drugs azaserine is an antibiotic that inhibits glutamine amidotransferases fluorouracil is changed in the body to fluorodeoxyridylate (FdUMP) which in turn binds to thymidylate synthase inhibiting it o Examples of mechanism-based inactivation methotrexate (amethopterin) and aminopterin are competitive inhibitors of dihydrofolate reductase o Fluorouracil, methotrexate and aminopterin are important anticancer agents; trimethoprim- is an antibiotic with a 35 higher affinity for bacterial DHFR 36 Pyrimidine salvage pathways • Mammalian cells reutilize a small amount of free pyrimidine bases • Pyrimidine phosphorylase can synthesize uridine and cytidine (through the reaction of uracil and cytosine with ribose-1-P) but it cannot use thymine A ribonucleoside containing thymine is not normally synthesized in the body Instead, thymine will react with deoxyribose-1-P to form thymidine • The nucleosides uridine, cytidine, thymidine and deoxycytidine are phosphorylated to their respective nucleotides Thymidine kinase (TK) levels increase significantly during the S phase of the cell cycle 37 Pyrimidine catabolism • The pyrimidine ring can be degraded unlike that of purines • Cytosine is degraded through uracil Cytidine and deoxycytidine are first deaminated to uridine and deoxyuridine or dCMP (deoxycytidylate) can be deaminated to dUMP (deoxyuridylate) and 5’-nucleotidase converts dUMP to 38 uridine • Uridine and cytidine are changed to uracil and cytosine by pyrimidine phosphorylase • dTMP changed to thymine by the successive actions of 5’-nucleotidase and nucleoside phosphorylase • In addition to CO2 and NH4+ (which enters the urea cycle), the degradation of uracil gives β-alanine and thymine gives β-aminoisobutyrate β - alanine and β-aminoisobutyrate can be excreted Or β - alanine can further be processed to malonylCoA and β-aminoisobutyrate to methylmalonyl-CoA In some cases β-aminoisobutyrate is produced in excessive amounts – genetic predisposition (Chinese or Japanese ancestry) and leukemia (massive cell 39 destruction) 40