5.2 REACTIONS OF THE CARBONYL GROUPv2
advertisement

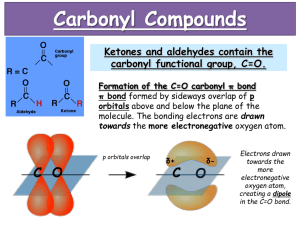
REACTIONS OF THE CARBONYL GROUP IN ALDEHYDES AND KETONES L.O.: The mechanism of nucleophilic addition reactions of carbonyl compounds How carbonyl compounds react when oxidised or reduced. A test for an aldehyde using Fehling’s solution. a) In a clean test tube mix together equal volumes of Fehling's solution A and Fehling's solution B. The resultant Fehling's test reagent should be a clear dark blue solution. b) Add 5 drops of this test reagent to 1 cm3 of sodium carbonate solution in a test tube containing a few antibumping granules and then add 1 cm3 of ethanal (or propanal) to this same test tube. c) Warm the test tube gently for approximately two minutes in a beaker half filled with hot water and then gradually bring the beaker of water to boiling and maintain this temperature for a few minutes. d) Using the test tube holder, carefully lift the test tube out of the boiling water and allow its contents to stand for several minutes. Tollens’ silver mirror test Prepare a sample of Tollens’ reagent by adding 5 drops of sodium hydroxide solution to 2 cm3 of silver nitrate solution in a test tube. To this test tube add just enough dilute ammonia solution to dissolve the brown precipitate completely. Using a beaker of hot water (50 oC to 60 oC), gently warm approximately 5 cm3 of this test reagent in a test tube. Add 10 drops of the aldehyde to the warmed test reagent in the test tube. Wait a few minutes and note what happens. Carbonyl groups consists of a carbonoxygen double bond The bond is polar due to the difference in electronegativity CARBONYL COMPOUNDS - NUCLEOPHILIC ADDITION Mechanism Nucleophilic addition STEP 1 Step 1 CN¯ acts as a nucleophile and attacks the slightly positive C One of the C=O bonds breaks; a pair of electrons goes onto the O CARBONYL COMPOUNDS - NUCLEOPHILIC ADDITION Mechanism Nucleophilic addition STEP 1 STEP 2 Step 1 CN¯ acts as a nucleophile and attacks the slightly positive C One of the C=O bonds breaks; a pair of electrons goes onto the O Step 2 A pair of electrons is used to form a bond with H+ Overall, there has been addition of HCN CARBONYL COMPOUNDS - NUCLEOPHILIC ADDITION Mechanism Nucleophilic addition STEP 1 STEP 2 Step 1 CN¯ acts as a nucleophile and attacks the slightly positive C One of the C=O bonds breaks; a pair of electrons goes onto the O Step 2 A pair of electrons is used to form a bond with H+ Overall, there has been addition of HCN CARBONYL COMPOUNDS - NUCLEOPHILIC ADDITION ANIMATED MECHANISM CARBONYL COMPOUNDS - NUCLEOPHILIC ADDITION Watch out for the possibility of optical isomerism in hydroxynitriles CN¯ attacks from above CN¯ attacks from below Many reducing agents will reduce ketones and aldehydes to alcohols. NaBH4 (sodium tetrahydroborate(III) ) generates the nucleophile H-, the hydride ion. Write the mechanism of the reaction of a ketone/aldehyde with H-. Will NaBH4 react with an alkene? NO! H- is repelled by the electron density of C=C. CH2 = CHCHO + 2[H] ———> CH2 =CHCH2OH CARBONYL COMPOUNDS - REDUCTION Example COMPOUND X What are the products when Compound X is reduced? H2 NaBH4 CARBONYL COMPOUNDS - REDUCTION Example What are the products when Compound X is reduced? COMPOUND X H2 NaBH4 C=O is polar so is attacked by the nucleophilic H¯ C=C is non-polar so is not attacked by the nucleophilic H¯ Oxidation Fehling test (Cu2+). Silver mirror test (Ag+) Is it more difficult to oxidise a ketone than an aldehyde? Why THE SILVER MIRROR TEST Tollen’s Reagent contains [Ag(NH3)2 ]+ When an aldehyde is warmed with Tollen’s reagent, metallic silver is formed. Aldehyde is oxidised to carboxylic acid and Ag+ reduced to metallic silver RCHO + [o] -> RCOOH oxidation [Ag(NH3)2]+ + e- -> Ag + 2NH3 reduction clip FEHLING’S SOLUTION It contains a copper(II) complex ion giving a blue solution. On warming, it will oxidise aldehydes. The copper(II) is reduced to copper(I) and a red precipitate of copper(I) oxide, Cu2O, is formed. Ketones do not react with Tollen’s Reagent or Fehling’s Solution video 2