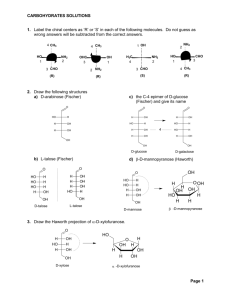
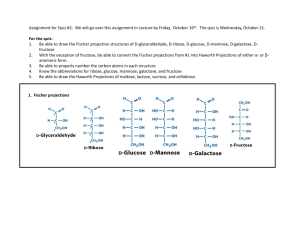
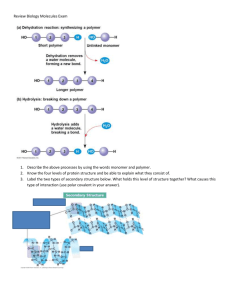
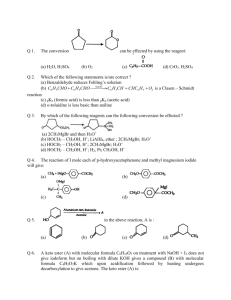
25_13_16.html
advertisement

25.13 Glycosides Glycosides Glycosides have a substituent other than OH at the anomeric carbon. Usually the atom connected to the anomeric carbon is oxygen. Example HOCH2 O HO HO OH OH D-Glucose Linamarin is an O-glycoside derived from D-glucose. HOCH2 HO HO O CH3 OCC OH CH3 N Glycosides Glycosides have a substituent other than OH at the anomeric carbon. Usually the atom connected to the anomeric carbon is oxygen. Examples of glycosides in which the atom connected to the anomeric carbon is something other than oxygen include S-glycosides and Nglycosides. Example Adenosine is an Nglycoside derived from D-ribose NH2 N HOCH2 H O H OH OH H H OH D-Ribose N N HOCH2 N H O H H OH H OH Adenosine Example HOCH2 O HO HO OH OH D-Glucose Sinigrin is an HOCH2 S-glycoside HO derived from HO D-glucose. O NOSO3K SCCH2CH OH CH2 Glycosides O-Glycosides are mixed acetals. O-Glycosides are mixed acetals CH O O OH H CH2OH hemiacetal O-Glycosides are mixed acetals CH O O OH hemiacetal H CH2OH ROH O OR H acetal Preparation of Glycosides Glycosides of simple alcohols (such as methanol) are prepared by adding an acid catalyst (usually gaseous HCl) to a solution of a carbohydrate in the appropriate alcohol. Only the anomeric OH group is replaced. An equilibrium is established between the a and b-glycosides (thermodynamic control). The more stable stereoisomer predominates. Preparation of Glycosides CH O H OH HO H H OH H OH CH2OH D-Glucose HOCH2 HO HO CH3OH O OCH3 OH HCl + HOCH2 HO HO O OH OCH3 Preparation of Glycosides HOCH2 Methyl b-D-glucopyranoside HO HO O OCH3 OH + Methyl a-D-glucopyranoside (major product) HOCH2 HO HO O OH OCH3 Mechanism of Glycoside Formation HOCH2 HO HO •• • O• OH OH HCl HOCH2 HO HO •• O •• carbocation is stabilized by lone-pair donation from + oxygen of the ring H OH Mechanism of Glycoside Formation HOCH2 •• • O• HO HO OH HOCH2 HO HO CH3 HOCH2 O HO O •• + HO + H OH + O H3C •• H CH3 •• •• O •• O •• H + OH H Mechanism of Glycoside Formation HOCH2 HO HO •• • O• OH HOCH2 HO HO CH3 HOCH2 O HO O •• + HO + H + –H OH + O H3C •• H HOCH2 •• O •• OH HO HO •• OCH3 + •• •• O •• OH •• OCH 3 •• 25.14 Disaccharides Disaccharides Disaccharides are glycosides. The glycosidic linkage connects two monosaccharides. Two structurally related disaccharides are cellobiose and maltose. Both are derived from glucose. Maltose and Cellobiose HOCH2 HOCH2 O HO 1 HO a OH O O OH 4 HO Maltose OH Maltose is composed of two glucose units linked together by a glycosidic bond between C-1 of one glucose and C-4 of the other. The stereochemistry at the anomeric carbon of the glycosidic linkage is a. The glycosidic linkage is described as a(1,4) Maltose and Cellobiose HOCH2 HOCH2 O HO 1 HO b OH O O OH 4 HO Cellobiose OH Cellobiose is a stereoisomer of maltose. The only difference between the two is that cellobiose has a b(1,4) glycosidic bond while that of maltose is a(1,4). Maltose and Cellobiose Maltose Cellobios e Cellobiose and Lactose HOCH2 HOCH2 O HO 1 HO b OH O O OH 4 HO Cellobiose OH Cellobiose and lactose are stereoisomeric disaccharides. Both have b(1,4) glycosidic bonds. The glycosidic bond unites two glucose units in cellobiose. It unites galactose and glucose in lactose. Cellobiose and Lactose HOCH2 HOCH2 O HO 1 HO b OH O O OH 4 HO Lactose OH Cellobiose and lactose are stereoisomeric disaccharides. Both have b(1,4) glycosidic bonds. The glycosidic bond unites two glucose units in cellobiose. It unites galactose and glucose in lactose. 25.15 Polysaccharides Cellulose Cellulose is a polysaccharide composed of several thousand D-glucose units joined by b(1,4)-glycosidic linkages. Thus, it can also be viewed as a repeating collection of cellobiose units. Cellulose Four glucose units of a cellulose chain. Starch Starch is a mixture of amylose and amylopectin. Amylose is a polysaccharide composed of 100 to several thousand D-glucose units joined by a(1,4)-glycosidic linkages. 25.16 Cell-Surface Glycoproteins Glycoproteins Glycoproteins have a protein backbone to which carbohydrates are attached. One example is the cell-surface glycoprotein that is recognized by the influenza virus. O OH HO CO2H CH3CNH O O O PROTEIN OH CH2O O NHCCH3 HO OH CH2OH Glycoproteins A second example is the group of glycoproteins that define the various human blood groups. Human-Blood-Group Glycoproteins HO R CH2OH O O O O H3C O OH HO HO N-Acetylgalactosamine polymer PROTEIN Type O: R = H HO Type A: R = CH2OH O HO CH3CNH O HO Type B: R = CH2OH O HO OH