Carbohydrates
advertisement

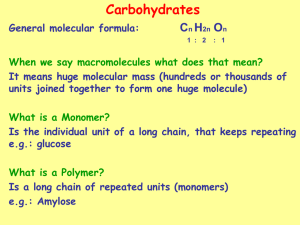
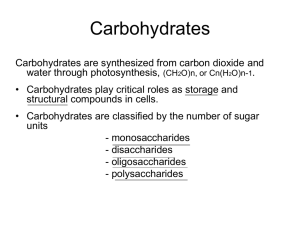
Carbohydrates Carbohydrate • hydrate of carbon – Cn(H2O)m • Glucose -blood sugar- C6H12O6 or C6(H2O)6 • Sucrose -table sugar- C12H22O11 or C12(H2O)11 • Not all carbohydrates have this exact form – old habits die slow or sometimes never at all Monosaccharides • Formula CnH2nOn • One carbon is either an aldehyde or ketone • The suffix ose indicates that the molecule is a carbohydrate • Use prefix to indicate number of carbons tri , tetr, pent, hex • Aldose – contain an aldehyde group • Ketose – contain a ketone group Monosaccharides • Aldohexoses Aldohexoses aldehyde function six carbons carbohydrates or saccharides other examples: ketotetrose, aldotriose, ketopentose Fischer Projections • Emil Fischer (late 1800’s) – Side groups come out of the plane (towards you) – Vertical groups go back away from you CHO CHO CHO HO H H OH H HO H H OH HO CH 2OH A CH 2OH B CHO OH H CH 2OH C HO H H OH CH 2OH D Naming Aldotriose and aldotetrose Remember ET goes home left at T Naming AldoPentose RAXL – Ribose, Araginose, Xylose, Lyxose Right – Top – Middle – Top/Middle Naming Aldohexoses • All Altrose Gladly Make Gum in Gallon Tanks • • • • Bottom – all right 2 up – 4x4 3 up – 2x2 like Noahs Ark 4 up – alternate R/S and D/L • R = D - dextrorotatory • S = L - levarotatory • D – A monosaccharide with the Penultimate OH group on the right in a Fischer Projection • L - A monosaccharide with the penultimate OH group on the left in a Fischer Projection OK, so what’s a penultimate???? Ketoses * • Note: triose tetrose The ketone is located on carbon #2 pentoses Penultimate is the next To last carbon hexoses * Amino Sugars • Contain an NH2 group instead of an OH • 1st three are common in nature Cyclic structure of monsacharides • It’s the hemiacetal reaction all over • We draw them as Haworth Projections • Practice practice practice From yahoo images Reactions of Monosacharides • Practice From yahoo images The two Most Significant Sugars •Aldose -D-glucose -The most important monosaccharide -White solid -Formula C6H12O6 - Sugar used in our bodies • Ketose – D-Fructose – Known as “fruit sugar” – Found combined with glucose in the disaccharide sucrose CH 2OH O H 1 C H HO H H 2 C 3 C 4 C O OH H OH HO OH OH OH CH 2OH CH 2 OH D-fructose 5 C 6 Multiple Sugars • • • • Monosaccharides – Single Carbohydrate unit Disaccharides - two monosaccharides combined Oligosaccharides - three to ten monosaccharides Polsaccharide – More than ten monoscharides Three Disaccharides • Sucrose • Lactose • Maltose Disaccharides • Sucrose * D-glucose – A disaccharide O – One D-glucose and D-fructose one D-Fructose – Connected by two anomeric carbons: C-1 on glucose and C-2 on fructose linkages: linkage on glucose and linkage on fructose Disaccharides * • Sucrose D-glucose – Anomeric C are tied-up O on both sugars D-fructose – No oxidation can occur no hemi-acetals – Sucrose is a non-reducing sugar – Hydrolyzed by enzymes to form a mixture of glucose and fructose - “invert sugar” Disaccharides • Sucrose * HO O HOCH2 HO -D-glucose OH -C-1 O O HOCH2 -C-2 HO CH2 OH OH -D-fructose Disaccharides • Lactose * – A disaccharide D-galactose O – One D-galactose and one D-glucose – Connected by an linkage between D-Galactose C-1 and D-Glucose C-4 – Known as an -1,4 linkage – Found in mammalian milk D-glucose Disaccharides HO O HOCH2 HO + OH -D-galactose O HOCH2 HO OH OH -D-glucose OH HOCH2 HO O -1,4 linkage OH O Galactose Lactose HOCH2 HO O OH OH Disaccharides • Maltose * – A disaccharide D-glucose O D-glucose – Two D-glucose monomers – Connected by an linkage between C-1 and C-4 – Known as an -1,4 linkage (two D-glucose molecules) – An ingredient in most syrups – “Malt sugar” Disaccharides HO HO HOCH2 + O HO -D-glucose HOCH2 OH O HO OH -D-glucose OH OH HO HOCH2 O HO OH -1,4 linkage Maltose O HOCH2 HO O OH OH Polysaccharides • Starch - Amylose * – Many units of -D-glucose – Linkages are -1,4 (same as Maltose) – Between 1000-2000 glucose units (polyglucose) – Random coils or helix Polysaccharides • Starch - Amylopectin * – Also many units of -D-glucose – Linkages are -1,4 and -1,6 – Lots of branching – 20 to 25 glucose monomers in the straight chain and then branching – A total of 105 to 106 glucose molecules – Use Iodine (I2) to test for starches Polysaccharides • Glycogen* – Animal energy storage (about 400 g in us) – -D-glucose polymer – Similar to amylopectin but smaller chains – Linkages are -1,4 and -1,6 (branching) – 10 to 20 glucose monomers in the straight chain and then branching – A total of 105 to 106 glucose molecules Polysaccharides • Cellulose* – Linear polymer of D-glucose – Linkages are -1,4 ! – The most abundant molecule in living tissues – Cotton is about 95% cellulose – 300 to 3000 glucose units – Form fibrous rods Polysaccharides • Cellulose* – We cannot digest cellulose • glucose linkages! • Many bacteria and fungi have necessary enzyme • Ruminant mammals carry these bacteria • Termites also have necessary microorganisms HOCH2 HOCH2 HOCH2 ( O OH Starch O OH O OH O OH HOCH2 ) OH alpha linkage ( O OH Cellulose OH O ) OH beta linkage