Biomass Fundamentals
advertisement

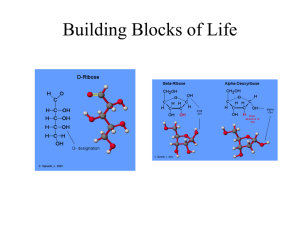
Biomass Fundamentals Modules 6-11: Carbohydrates: a major building block for biomass A capstone course for BioSUCCEED: Bioproducts Sustainability: a University Cooperative Center of Excellence in EDucation The USDA Higher Education Challenge Grants program gratefully acknowledged for support This course would not be possible without support from: USDA Higher Education Challenge (HEC) Grants Program www.csrees.usda.gov/funding/rfas/hep_challenge.html Emil Fischer: Father of Carbohydrate Chemistry • Prof. of Organic Chemistry University of Berlin (1852-1919) • Known for his monumental work on configuration of sugars • Also worked on aminoacids, proteins, indoles & stereochemistry • As a Grad. student he discovered phenylhydrazine Nobel prize for Chemistry 1902 What is a carbohydrate? • Carbohydrates are polyhydroxyl compounds general formula Cn(H2O)n “Hydrates of Carbon” • All contain hydroxyl groups -OH primary -CH2OH and secondary =CHOH Quiz M6/11.1 1. What is a saccharide? • A carbohydrate possessing the empirical formula, Cm(H2O)n, or hydrates of carbon analogous to inorganic hydrates (e.g., Fe2 (SO4)3*6H2O) • Shown at left is open chain form (Fischer Projection formula) of D-Glucose, isomer in which the hydroxy on C5 is RIGHT D-Glucose is a stereospecific form of glucose having the orientation specified by the Fisher Projection formula shown at left. Abundance Quiz M6/11.2 1. • Carbohydrates are the most abundant living biomaterial (a substance derived from living systems) on the planet • They comprise the bulk of plants, while constituting a significant portion of the cellular membrane of animals (yet, lesser than proteins and lipids) • They are however, a significant source of stored energy Typical carbohydrates • D-glucose, D-fructose • Sucrose: common table sugar that is a disaccharide synthesized from dehydration of D-glucose & D-fructose • Lactose: common milk sugar that is a disaccharide of D-glucose & D-galactose Structure of carbohydrates Based on glyceraldehyde Quiz M6/11.1 1. Common carbohydrates ESSAY At this point, describe in a page or less, how XXX contributes to your lifestyle or how you would like it to Common carbohydrate: sucrose • Dimer • Disaccharide • Indispensable to our way of life! • Notice -acetal linkage The linkage between glucose and fructose responsible for the formation of this dimer known as sucrose is known as a glycosidic bond. Nomenclature Quiz M6/11.4 Monosaccharides - characterized in terms of the number of carbons Example # of Carbons Name 3 triose 4 tetrose xylose 5 pentose glucose 6 hexose glyceraldehyde etc 1. Nomenclature: part II CHO H OH HO H CHO H HO H OH H OH HO H H OH HO H CH2OH d-glucose CH2OH l-glucose Shown above are two forms of a generic amino acid (where R = carbon-containing groups) that are said to be “enantiomeric.” Nomenclature: part III 1 CHO H OH 2 HO H H 3 H H OH 4 4 HO HO 5 OH 6 CH2OH 4 HO HO 5 H OH 2 3 H OH H O 1 H D glucose Fisher Projection H OH 6 OH 6 5 2 3 H H H O OH OH 4 6 HO HO OH 1 H H Chair Conformation -D glucopyranose 5 3 H H H O 2 H OH 1 OH -D glucopyranose • C1: Anomeric Carbon • α : Axial configuration (vertical to the seat of the chair) • β : Equatorial configuration (parallel to the seat of the chair) Most Important Chemical Consideration of Sugars Consider the anomeric carbon! The aldehyde on the one position can be nucleophilically attacked by any of the hydroxyls! Hemiacetalization Concept Key to Carbohydrate Ring Structures Nomenclature of Carbohydrates • D, L Defines the configuration at C5 D has the OH at Right in Fischer projection L has the OH at Left in Fischer projection • Gluco defines the configuration of the OH at C2, C4, C5. These OH’s are on same side while the C3-OH is opposite to others • α,β defines the configuration of the OH at C1, the anomeric carbon • Pyran indicates 6 member ring size • Furan indicates 5 member ring size Examples follow In Glucuronic acid C2, C4, C5 OH’s are on same side H C H HO H H H O OH C H O OH H HO H OH HO H OH H CO2H glucuronic acid OH CO2H galacturonic acid Alditols • In Mannitol C2, C4, C5 OH’s are not at same side in Fisher Projection CH2OH CH2OH HO HO H H H OH H OH H OH HO H H OH CH2OH CH2OH Mannitol Xylitol Conformations Anomers CH2OH O OH OH OH OH OH -D glucopyranose [a] 25 D CH2OH O OH -D glucopyranose +19o For aged solutions [a] OH OH +112o 25 Rotations of Fresh Solutions = +52.7o D Reason: Mutarotation is the best evidence for the cyclic hemiacetal structure of D-(+)-glucose Monosaccharides,Hemiacetal Formation II CH2OH H C C H OH HO CH2OH O .. H H C C C H OH O H H C C H OH H C C H OH HO O OH C H C5 OH attacks aldehyde giving a pyranose ring (6 member structure) CH2OH C H O .. C H OH HO CH2OH H H HO C C C H OH H O H C H O C OH C OH H C C H OH H C4 OH attacks aldehyde giving a furanose ring (5 member structure) -D glucofuranose CH2OH HO O OH -D glucopyranose Mutarotation CH2OH O OH OH OH OH Ring closure between C1 OH and C4 -OH CHO OH CH2OH OH OH CHO OH OH CH2OH O OH HO OH H HO H H OH H OH OH CH2OH D glucose CH2OH OH CHO OH OH OH Ring closure between C1 and C5 -OH CH2OH O OH OH OH OH -D glucofuranose OH -D glucopyranose Hemiacetalization Concept Key to Carbohydrate Ring Structures • Oligosaccharides – consist of several monosaccharide residues joined together with glycosidic linkages – di, tri, tetrasaccharides (depending on the number of monosaccharides) – up to 10 - 20 monosaccharides (depending on analytical techniques i.e GC vs LC/MS) • Polysaccharides – refer to polymers composed of a large number of monosaccharides linked by glycosidic linkages ex. Cellulose Cellobiose CH2OH HO HO O OH CH2OH OH HO O O CH2OH anhydroglucopyranose unit O HO O OH OH HO O OH O CH2OH n = 1 -5000 oxygen bridge (ether-type or glycosidic bond) Cellulose -D-anhydroglucopyranose units linked by (1,4)-glycosidic bonds 6 HO HO OH CH2OH O Non-Reducing End-Group CH2OH 4 O HO 5 3 HO O 2 OH OH 3' O 1 4' 5' 2' CH2OH O 6' CH2OH 1' O O HO n OH OH Reducing End-Group (potential aldehyde) Polysaccharides Polysaccharides are polymers composed of many monosaccharide units linked by glycosidic bonds The glycosidic bond can can have either the α or a β-configuration and be joined to any of the hydroxyl groups at C-2, C-3, C-4 or C-6 The chain can either be Linear or Branched – branches can be single monosaccharide units, chains of two or more units, or chains of a variable number of units Polysaccharides Polysaccharides can be divided into two classes – Homopolysaccharides • consist of only one kind of monosaccharide ex cellulose – Heteropolysaccharides • consist of two or more kinds of monosaccharides ex galactoglucomannans Homopolysaccharides Homopolysaccharides can be further divided by the type(s) of glycosidic linkages Homolinkages - either an α or a β configuration to a single position (exclusive of any branch linkages) •that is a single kind of monosaccharide linked by one type of bond α-14, β-14, and so on Heterolinkages - a mixture of a- and bconfigurations and/or mixture of positions •usually have a definite pattern for the arrangement of the linkages Heteropolysaccharides Heteropolysaccharides can have the same kind of linkage diversity as with homopolysaccharides, but now associated with one or more of the different kinds of monosaccharide units – infinite degree of diversity of structure Polysaccharides Polysaccharides can not only have different sequences of monosaccharide units, but also different sequences of glycosidic linkages and different kinds of branching – a very high degree of diversity for polysaccharides and their structurefunction relationships Plant Polysaccharides The conformation of individual monosaccharide residues in a polysaccharide is relatively fixed, however, joined by glycosidic linkages, they can rotate to give different chain conformations. 1,4 glycosidic linkage OH O HO O HO O HO OH O HO HO OH HO HO O O 1,6 glycosidic linkage HO HO O O HO O Plant Polysaccharides The different kinds of primary structures that result in secondary and tertiary structures give different kinds of properties – water solubility, aggregation and crystallization, viscosity, gelation, etc. Polysaccharides have a variety of functions – Storage of chemical energy in photosynthesis – Inducing Structural Integrity in plant cell walls Starch Starch is composed completely of D-glucose – found in the leaves, stems, roots, seeds etc in higher plants – stores the chemical energy produced by photosynthesis Most starches are composed of two types of polysaccharides - amylose and amylopectin – amylose - a mixture of linear polysaccharides of D-glucose units linked -(1-4) to each other • between 250-5,000 glucose residues The Components of Starch O HO OH O OH O HO OH O OH O (1-4) HO Amylose OH HO O O OH HO O OH O Amylopectin – Amylopectin - a mixture of branched polysaccharides of Dglucose units linked -(1-4), with ~ 5% -(1-6) branch linkages • between 10,000-100,000 glucose residues OH O OHO OH O HO OH O OH O OH O OH O HO O HO OHO HO (1-4) OH O (1-6) O OH O HO OH O HO O Starch Polymer Components Amylose Amylopectin (1 residue in every 20 is 16 linked to branch off) The Components of Starch Amylose O HO Amylopectin OH O OH O OH O HO OHO OH O OH O HO OH O OH O OHO HO OH O HO OH O (1-4) HO OH HO O HO (1-4) O OH O (1-6) O OH O HO OH O O OH HO O OH O HO O OH O Starch tertiary structure (Helix) Building Blocks of Life Hemiacetalization Concept Key to Carbohydrate Ring Structures Fructose is Bane of Our Civilization • • • • • • Glycoproteins (biomaterial) from food sugars are sticky – adhere to teeth enamel Streptococcus mutans also adhere to biomaterial The glucose of the sucrose is polymerized by glucosyl transferase (enzyme of bacteria) to form plaque Fructose from the above hydrolysis is released Bacteria needs anaerobic conditions which are partially supprted by plaque to hydrolyze fructose for energy Ca3(PO4)2 + CH3CH2(OH)COOH CaHPO4 + Ca+2 loss of enamel Alditols: Reduction Products Reduced aldehyde end-group D-Mannitol Aldaric Acids: Oxidation Products • Above is oxidation product of D-galactose • Galactaric acid • Why is it not labeled as “D- ?” Sorbitol (D-Glucitol) It is a polyol discovered in the berries of the mountain ash in 1872 commercially produced by the hydrogenation (reduction) of glucose). It does not lead to tooth decay. WHY? 60% as sweet as sucrose with 1/3 fewer calories Good sugar substitute Relative Sweetness COMPOUND RELATIVE SWEETNESS Lactose D-Galactose Maltose D-Glucose Sucrose D-Fructose Sodium cyclamate (procarcinogen) Aspartame Sodium saccharin (possible carcinogen) Sucralose (brand name = Splenda) Neohespiridin dihydrochalcone 5-nitro-2-propoxyaniline 0.2 0.3 0.3 0.7 1.0 (Standard) 1.7 30 190 500 600 1,000 4,000 Glucose Derivative Spotlight: Glucosamine Nitrogen • Found in mucin, a glycoprotein constituent of saliva • Plays important role in production, maintenance, and repair of cartilage (prevents bone ends from rubbing) • Used in Europe since 1980s to “offset” onset of osteoarthritis • USA is now touting its benefits (perform search – 906K hits) Most Important Chemical Consideration of Sugars Consider the anomeric carbon! The aldehyde on the one position can be nucleophilically attacked by any of the hydroxyls! Important Oligosaccharides • ,-tetrahalose: glucose disaccharide found in the circulatory systems of insects; energy for insect eggs, larvae, and pupae. Found in fungi & yeasts • Raffinose: widely distributed in plants at low concentration – 1:1:1 D-galactose:Dglucose:D-fructose (galactose+sucrose in which it is on C6 of glucose moiety) Starch (Amylose) • High MW polyglucoside that is plant energy • Can be hydrolyzed to specific oligosaccharides • -Amylase (saliva & pancreatic juice) converts it to maltose and glucose • -Amylase (in plants only) converts it to maltose – NOTE: malt from barley is its common source to BREW BEER Starch, Part Deux • Noncrystalline • Partially water soluble • 25% amylose (linear polysaccharide), 75% amylopectin (branched polysaccharide) Starch Polymer Components Amylose Amylopectin (1 residue in every 20 is 16 linked to branch off) Homework Questions • Draw structure of raffinose by only consulting these notes! • Provide an explanation for the “sweetness” of saccharides – you may consult the literature • Using your explanation, design a molecule that may compete with commercial sugars for market share! Article of Interest for Discussion • “Chemically modified chitin and chitosan as biomaterials” – Sashiwa, H.; Aiba, S.-I. Prog. Polym. Sci. 29 (2204) 887-908. • http://www.sciencedirect.com/science?_ob=MImg&_imagekey=B6TX2-4CNJ8S2-16&_cdi=5578&_user=290868&_orig=search&_coverDate=09%2F30%2F2004&_qd=1&_sk =999709990&view=c&wchp=dGLbVzbzSkzS&md5=e9e66537b26f5f36324572d9f01635b8&ie=/sdarticle.pdf • Chitosan-derivatized -CD (2-position) • Slow radiative iodine release in blood of rats • Decontamination of textile dye-laden waters • Absorbent matrix • Positive cholesterol interaction Structure of Starch • Unbranched chains • 500-20K -(14)-Dglucose units • Extended shape has possible 7-22 nm hydrodynamic radius • Usually forms stiff lefthanded single helix Hydrogen Bonding in Amylose • Single helical amylose has H-bonding between O2 O6 atoms on outside • Syneresis (release of water) occurs • Double-stranded crystallites formed resistant to amylase • Fairly hydrophobic & low solubility • Interior is like cyclodextrins – hydrophobic Cyclodextrins (CDs) • Cyclic oligomers of -Dglucopyranose • Glycosidic bonds • Discovered in 1891 by Villiers • Synthesized by the enzymatic conversion of amylose & selective precipitation Cavity volume: 0.174, 0.262, & 0.472 nm2 for , , & -CDs, respectively CD Formation Precipitating Agent Yield (%) -CD 1-decanol 40 -CD toluene 50-60 -CD Cyclohexadec8-en-1-ol 40-50 Interesting Properties • Formation of inclusion (host/guest) complexes with lipophilics – -CD: aliphatic chains – -CD: small aromatics (toluene) – -CD: larger molecules • Low toxicity • Biodegradable From Frömming and Szejtli: Cyclodextrins in Pharmacy, Kluwer, Acad. Press, Dordrecht, 1994. Cyclodextrin Derivatives of Pharmaceutical Interest OR HO OH O OH O O O OH OH HO OR O RO HO O OH O OR O HO O RO HO OH O HO O O OH OH O HO OR Cyclodextrin R DS HPCD CH2CHOHCH3 0.6 SBECD (CH2)4SO3- Na+ 0.9 RMCD CH3 1.8 Also the CD derivative: HPCD Some cyclodextrin-containing products Cyclodextrin Product Trade name CD PEG1 iv infusion Prostavastin (Eur.), Caverject (USA) CD Piroxicam tablets Brexin (Eur.) HPCD Intraconazole oral solution and iv soln. Sporanox (Eur. and USA) SBECD Ziprasidone maleate im solution Zeldox (Eur.), Geodon (USA) RMCD Estradiol nasal spray Aerodiol (Eur.) OP-1206 tablets Opalmon (Japan) Diclofenac sodium eye drop solution Voltaren (EUR.) CD HPCD World-wide there are close to 30 cyclodextrin containing pharmaceutical products on the market and most of them are marketed in more than one country. Almost half of them contain the natural CD. What are cyclodextrins used for? • To increase aqueous solubility of drugs. • To increase chemical stability of drugs. • To enhance drug delivery to and through biological membranes. • To increase physical stability of drugs. • To convert liquid drugs to microcrystalline powders. • To prevent drug-drug and drug-excipient interactions. • To reduce local irritation after topical or oral administration. • To prevent drug absorption into skin or after oral administration. And so on. Cyclodextrin complexation: conventional wisdom -Cyclodextrin: Seven -1,4-linked glucopyranose units form a cone with a hydrophilic outer surface and a lipophilic cavity in the center. Complex Formation From Frömming and Szejtli: Cyclodextrins in Pharmacy Kluwer Acad. Press, Dordrecht, 1994. Cyclodextrin Complexes Doxorubicin-CD complex Aspirin-CD complex Why are cyclodextrins better than organic solvents? • Frequently less irritating after iv and im injection. • Frequently less toxic. • The drug does not precipitate after iv injection. • Can be used in solid dosage forms. Homework Questions • Describe why CD is in “Fabreeze” (P&G product) and how you think it works. • How do K1:3 complexes form? Give an example.