Temperature, Heat, and Expansion
advertisement
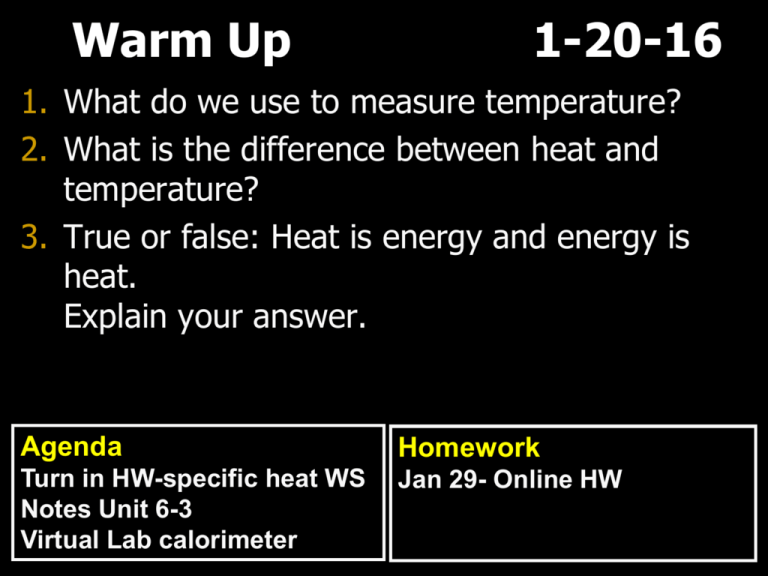
Warm Up 1-20-16 1. What do we use to measure temperature? 2. What is the difference between heat and temperature? 3. True or false: Heat is energy and energy is heat. Explain your answer. Agenda Homework Turn in HW-specific heat WS Notes Unit 6-3 Virtual Lab calorimeter Jan 29- Online HW Answers: 1. Specific heat 2. O Kelvin 3. Endothermic 4. Exothermic 5. 4.18 6. Delta T 7. O oCelcius 8. 0.416 9. 63,536 Joules 10. 7.974 g 11. 0.2 12. 13,794 joules 13. 0.031 Answers: 14. 27, 912 joules 15. 58,050 joules 16. 12.096 grams 17. 15.01818 Celsius 18. Object B, lower specific heat means it requires less heat for temperature to increase Unit 6 - 3 Enthalpy Enthalpy • Enthalpy – the total quantity of heat in a reaction • Changes in enthalpy are measured through indicators (temperature) • Represented by ΔH Enthalpy Change • The sign of H indicates the direction of heat transfer. +H indicates heat is entering the system (endothermic) - H indicates heat has left the system (exothermic). Enthalpy Change • For reactions H = H (products) – H (reactants). Example: • 2H2 (g) + O2 (g) 2H2O(g) H = -483.6kJ Molar Heat Capacity • The quantity of heat required to raise the temperature of one MOLE of a substance by one degree Celsius or Kelvin Molar Heat Calculation • Q = n x ΔT x Cp n = moles Cp = molar heat capacity Example Problem • The molar heat capacity of CCl4 is 130.7 J/mol·C. What is the enthalpy change when 125g of CCl4 is heated from 10 °C to 32 °C. Convert to mole if given grams Calorimeter • An instrument for measuring the heat of a reaction during a chemical process $13,000 – $40,000 Specific Heat Lab Q gained by water = Q lost from the metal Assignments • Summary • Virtual calorimeter lab http://www.wiley.com/college/trefil/047 0118547/vdl/lab_calorimeter/ Homeroom -AP classes overview -Reflections and setting goals -Quote of the week 1-20-16 Homeroom 1-20-16 On a piece of line paper. Answer the following questions in complete sentences. 1.What were the high point(s) and low point(s) of last year? 2.List three things your did successfully in school or outside of school. 3.List several goals you want to achieve this semester