Exam 3 2012 - WordPress.com
advertisement

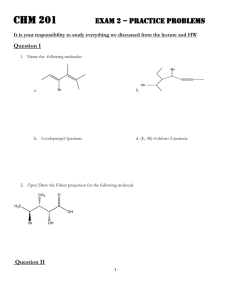
Boston College CH23101, Organic Chemistry I Professor Kelly Exam #3: December 7, 2012 Name: __________________________ (print, legibly please) Directions: There are 9 numbered pages not including this page (Note: several of these pages have considerable blank space on them). Be sure that NONE of the pages is missing. Scratch paper is attached and may be removed. Write answers in the spaces provided. Do not dawdle; budget your time wisely! 1. (12pts) Consider the molecule below (assume rapid rotation around single bonds): What will be approximately the chemical shift (ppm = δ) for hydrogens A?__________ For hydrogens B? __________ Which hydrogen(s) will give rise to a resonance furthest downfield ( = furthest to the left)? __________ Which, if any, of the six designated resonances (A-F) will appear as a singlet [if so give its/their letter(s), otherwise say "none"]? __________ As a triplet? __________ As a quartet? __________ As more peaks than a quartet? If yes, how many peaks? __________ Will any set of resonances (be it a singlet, doublet, triplet, or anything else) integrate for more than 3 hydrogens? If no, say "no." If yes, give the letter(s) for the hydrogens whose resonances will integrate to ≥4 hydrogens. __________ 2. (4pts) (a) Give on the line the name of: ______________________________________________________________________________ (b) Give the complete name for: ____________________________________________________________________________ 1 3. (6pts) (a) Put in the box the structure of a 4-carbon Grignard reagent that would give alcohol A after a standard Grignard reaction. (b) Give in the box the structure of a 5-carbon ketone that would give X after reaction with an appropriate Grignard reagent. 4. (4pts) Reaction of a 6-carbon secondary alcohol A gave the tertiary chloride B. Apparently, a rearrangement occurred. Give in the box a reasonable structure for A. 2 5. (4pts) Give in the box the letter of the one compound below that gives rise to the spectrum shown: 6. (3pts) The reaction below gives a mixture of two alkenes, one of which contains deuterium (D). Using a line drawing put in the box, including stereochemistry, the structure of the Dcontaining alkene. 3 7. (6pts) Indicate in the boxes how many resonances appear in the 13C (carbon!) NMR (with 1H decoupling) spectrum of the following compounds. 8. (4pts) We have not discussed the combustion of alkenes in class, but you should be able to figure out the answer to this question anyway. The combustion of ethene is shown by the equation below: For the four alkenes shown below, which will have the most negative ΔHcombustion (-20 is more negative than -10)? __________ The least negative ΔHcombustion? __________ 4 9. (12pts) Provide in the boxes the missing starting material, reagent(s), or organic product. Do 4, omit one, and indicate which. *If the starting material or product shown is chiral, use wedge/dashed notation to indicate the configuration of the corresponding product or starting material. If a racemic mixture is expected, say that. 5 10. (3pts) The reaction sequence below was suggested by the assigned Chemical Highlight 9-2. Chloroalcohol Z was subjected to the following sequence of reactions. Give the structure of the product P. 11. (6pts) Ignoring splitting, how many resonances would there be in the 1H (proton!) spectra of the two compounds shown? Put answers in the boxes. 12. (6pts) Reaction of dichloroether D with Na2S ( = 2Na+ and S2-) gave a mixture of A and B, both of which contain sulfur. The entire 1H NMR spectrum of A is just one singlet; the entire 1 H NMR spectrum of B is also just one singlet (not exactly the same chemical shift as in A), but B is twice the molecular weight of A. In the boxes, give the structures of A and B. A B 6 13. (6pts) Reaction of a Grignard reagent with an oxacyclopropane (also known as an epoxide) gave W upon work-up. In the boxes, place reasonable structures for the Grignard reagent and the oxacyclopropane. 14. (6pts) (a) Indicate on the line how many degrees of unsaturation a molecule with the formula C7H10O has. __________ (b) A compound with the formula C6H7BrS has one double bond. How many rings does it have (circle correct answer)? 0 1 2 3 4 -1 7 15. (4pts) An ester has the formula C4H7ClO2 and gives the 1H NMR spectrum below. Give its structure in the box. Note: The following problem may be time consuming. Think twice about whether working on it is the best investment of your time. 16. (4pts) Give in the box a reasonable structure for a compound that has the following properties: Contains 5 carbons The proton-decoupled 13C NMR spectrum contains four peaks Contains one bromine Has 2 degrees of unsaturation There may be more than one correct answer (and certainly many incorrect ones). Note: One more page to go! 8 17. (10pts) Synthesize two (indicate which one you want to omit) of the compounds below from the starting materials specified and any inorganic reagents you wish. Do not give mechanisms. Each of the syntheses will require two or more steps. A sample question and answer are provided. Write the structure of the product after each step. 9