Standard Reduction Potential for a Galvanic
advertisement

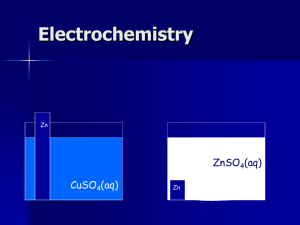
CONTENT OBJECTIVE WHAT THE HECK DO I NEED TO BE ABLE TO DO? I can make qualitative or quantitative predictions about galvanic (voltaic) cells based on half-cell reactions and potentials and analyze these cells to identify properties of the underlying redox reactions. Galvanic/Voltaic Cells 1. In Pre-AP Chemistry, we deal mostly with reactions that are spontaneous— meaning, they will happen on their own if all the “ingredients” are present. Electrochemistry 2. Galvanic cells use thermodynamicallyfavored (spontaneous) REDOX reactions to produce electrical energy via a flow of electrons (also known as Voltaic cells or batteries). a. In short: galvanic (voltaic) cells produce current! Electrochemistry Galvanic/Voltaic Cells 3. To use that current, we need to separate the place where oxidation is occurring from the place where reduction is occurring. Electrochemistry Galvanic/Voltaic Cells • QUICK REMINDERS* 1) Oxidation is LOSS of electrons OIL (LEO) Ex: Zn (s) Zn2+ (aq) + 2e2) Reduction is GAIN of electrons RIG (GER) Ex: Cu2+ (aq) + 2e- Cu(s) Electrochemistry Galvanic/Voltaic Cells B. Parts of the Galvanic Cell 1. Electron flow: ALWAYS through the wire from anode to cathode (alpha order) Electrochemistry Galvanic/Voltaic Cells Electrochemistry Galvanic/Voltaic Cells Electron (-) flow Electrochemistry Galvanic/Voltaic Cells B. Parts of the Galvanic Cell Say you have 2 solutions and you’re trying to make a galvanic cell: Zinc sulfate = ZnSO4(aq) Copper (III) sulfate Cu2(SO4)3 Ex: Zn (s) Zn2+ (aq) + 2eEx: Cu2+ (aq) + 2e- Cu(s) Electrochemistry Galvanic/Voltaic Cells B. Parts of the Galvanic Cell 2. anode (–): the electrode where oxidation occurs (may appear smaller over time) Oxidation: Zn (s) Zn2+ (aq) + 2e- Electrochemistry Galvanic/Voltaic Cells Electron (-) flow Electrochemistry Galvanic/Voltaic Cells Electron (-) flow Zinc anode (electrode) Zinc sulfate solution (oxidation) Electrochemistry Galvanic/Voltaic Cells B. Parts of the Galvanic Cell 3. cathode (+) : the electrode where reduction occurs (may appear larger over time) Reduction: Cu2+ (aq) + 2e- Cu(s) Electrochemistry Galvanic/Voltaic Cells Electron (-) flow Zinc anode (electrode) Zinc sulfate solution (oxidation) Electrochemistry Galvanic/Voltaic Cells Electron (-) flow Zinc anode (electrode) Zinc sulfate solution (oxidation) Copper cathode (electrode) Copper sulfate solution (reduction) Electrochemistry Galvanic/Voltaic Cells B. Parts of the Galvanic Cell 4. Salt bridge or (disk): bridge between cells whose purpose is to provide ions to balance the charge Electrochemistry Galvanic/Voltaic Cells Electron (-) flow Zinc anode (electrode) Zinc sulfate solution (oxidation) Copper cathode (electrode) Copper sulfate solution (reduction) Electrochemistry Galvanic/Voltaic Cells Electron (-) flow salt bridge Zinc anode (electrode) Zinc sulfate solution (oxidation) Copper cathode (electrode) Copper sulfate solution (reduction) Electrochemistry Galvanic/Voltaic Cells B. Parts of the Galvanic Cell 5. Voltmeter: measures the cell potential (emf or E°) in volts Electrochemistry Galvanic/Voltaic Cells Electron (-) flow salt bridge Zinc anode (electrode) Zinc sulfate solution (oxidation) Copper cathode (electrode) Copper sulfate solution (reduction) Electrochemistry Galvanic/Voltaic Cells Electron (-) flow voltmeter salt bridge Zinc anode (electrode) Zinc sulfate solution (oxidation) Copper cathode (electrode) Copper sulfate solution (reduction) Electrochemistry Voltaic Cells A typical cell all labeled looks like this. Electrochemistry C. Terms to remember through shortcuts • ca+hode: the cathode is + in galvanic/voltaic cells, and so the anode is negative (-) Electrochemistry C. Terms to remember through shortcuts • AN OX: oxidation occurs at the anode (may show mass decrease) • RED CAT: reduction occurs at the cathode (may show mass increase) (Combine that with remembering OIL RIG / LEO GER !) Anode = oxidation; oxidation is loss Reduction = cathode; reduction is gain Electrochemistry C. Terms to remember through shortcuts • FAT CAT: electrons in a voltaic/galvanic cell ALWAYS flow From the Anode To the CAThode And the cat gets fat! (cathode gains mass over time) “ANODE”-rexic! (anode loses mass over time) Electrochemistry D. Standard Reduction Potential for a Galvanic/Voltaic Cell (E°) ° cell, the metal 1. In a galvanic (voltaic) with the greater (more positive) reduction potential will be reduced! Electrochemistry D. Standard Reduction Potential for a Galvanic/Voltaic Cell (E°) ° come from a chart 2. Because the values of standard reduction potentials, you MUST REVERSE the sign of the E°of the oxidized species before adding to the E° of the reduced species. Electrochemistry D. Standard Reduction Potential for a Galvanic/Voltaic Cell (E°) 3. How to calculate ° the cell potential of a galvanic cell: Eoox = - Eored and Eocell = Eooxidation + Eoreduction Electrochemistry D. Standard Reduction Potential for a Galvanic/Voltaic Cell (E°) °oxidation-reduction 4. For a spontaneous (voltaic/galvanic cell) to occur, the overall cell potential must be positive. Electrochemistry • Example: Consider the half reactions shown below and the standard electrode reduction potentials that follow. Cu2+(aq) + 2 e- Cu(s) Zn2+(aq) + 2 e- Zn(s) Eo = +0.34 V Eo = -0.76 V Electrochemistry Cu2+(aq) + 2 e- Cu(s) Zn2+(aq) + 2 e- Zn(s) Eo = +0.34 V Eo = -0.76 V Which one has the GREATER (more positive) reduction potential? Cu2+ or Zn2+ (If it has the GREATER reduction potential, Eo, it has a greater desire for electrons and will be reduced!!!!) Electrochemistry Cu2+(aq) + 2 e- Cu(s) Zn2+(aq) + 2 e- Zn(s) Eo = +0.34 V Eo = -0.76 V Which one has the GREATER (more positive) reduction potential? Cu2+ or Zn2+ (If it has the GREATER reduction potential, Eo, it has a greater desire for electrons and will be reduced!!!!) Electrochemistry Keep the equation the same since it’s being reduced: Cu2+(aq) + 2 e- Cu(s) Therefore: Eored = +0.34 V Electrochemistry Zn2+(aq) + 2 e- Zn(s) Eo = -0.76 V Zinc has the LOWER reduction potential (Eo), so it will be oxidized. (Flip the sign of the Eo!) Flip the equation since it is being oxidized: Zn (s) Zn2+(aq) + 2eTherefore: Eoox = - Eored -(-0.76 V) = +0.76 V Electrochemistry Now calculate Eocell by plugging in the numbers: Eocell = Eoox + Eored = 0.34 + 0.76 = +1.10 V ****Galvanic (Voltaic) cells should ALWAYS have a positive o E cell value!!!!!!!!!!!!!**** Electrochemistry Take your half reactions, balance, and find the net ionic equation. Half reactions: Cu2+(aq) + 2 e- Cu(s) Zn (s) Zn2+(aq) + 2eNet ionic equation: Cu2+(aq) + Zn(s) Zn2+(aq) + Cu(s) Electrochemistry