Chapter 20: Electrochemistry Electrochemical Cells Anode
advertisement

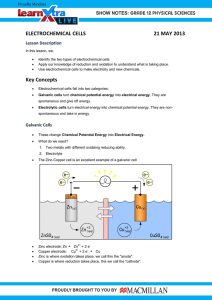
Chapter 20: Electrochemistry Electrochemical Cells Anode – electrode where oxidation occurs in any electrochemical cell Cathode – electrode where reduction occurs in any electrochemical cell Voltaic Cells (sometimes also called galvanic cells) Voltaic cells are spontaneous and used to generate electrical power An oxidation reaction takes place at the anode where chemicals give electrons to the electrode and the electrons then flow to the cathode where reduction occurs The original voltaic cell is credited to Alessandro Volta in the 1800s Volta made a pile of copper and zinc circular plates using sulfuric acid as his electrolyte The half reactions involved are: Oxidation: Zn → Zn2+ + 2e– Reduction: 2H+ + 2e– → H2 Since Volta stacked several cells together, he actually made the first battery His battery quickly lost power when running because the hydrogen that forms on the copper plates coats the copper and eventually prevents the solution from coming in contact with the copper electrodes In order to produce useful power, cells are usually separated into half-cells As a typical galvanic cell, consider a zinc–copper galvanic cell A zinc electrode is placed in a zinc sulfate solution in a beaker A copper electrode is placed in a copper sulfate solution in a second beaker Nothing will happen until the two electrodes are joined by a conductive wire Since Zn is more active than Cu, the Zn electrode (the negative anode) will begin to push electrons onto the copper electrode but the buildup of negative sulfate ions in the cathode half-cell will quickly stop the cell from working All working cells will require a complete circuit which is accomplished by adding a salt bridge A salt bridge could be as simple as a wet paper towel that has been soaked in a salt solution Most salt bridges are formed by a salt in a gel in a U shaped tube that bridges to two beakers Once the salt bridge completes the circuit, the cell will run until it runs out of zinc in the anode half-cell or until it runs out of copper ions in the cathode half-cell The half reactions involved are: Oxidation: Zn → Zn2+ + 2e– Reduction: Cu2+ + 2e– → Cu Here is a schematic of our Zn – Cu voltaic cell: from http://en.wikipedia.org/wiki/Galvanic_cell Chemists usually use a shorthand method to diagram a cell This shorthand always shows the oxidation half-cell then the reduction half-cell: Oxidation: reactant │ product ║ reactant │product :Reduction 2+ 2+ Zn │Zn ║ Cu │Cu The potential (voltage) a cell will give is calculated using the formula: The values for the reduction and oxidation potentials come from a table of standard reduction potentials like Table 20.1 on page 712 of your Glencoe Chemistry text Example: find the standard cell potential for a Zn │Zn2+ ║ Cu2+ │Cu cell 0.3419V 0.7618V = 1.1037 V Electrolytic Cells Electrolytic cells are forced to run in the opposite direction as a spontaneous voltaic cell This is usually done to: Recharge a voltaic cell Electroplate something (usually metal) with a protective metal like chromium Produce a useful chemical like sodium or chlorine gas Downs cell – an example of an electrolytic cell used to produce sodium metal and chlorine gas from molten sodium chloride from: http://en.wikipedia.org/wiki/File:DownsCellSchematic.svg Note that Sodium ions are reduced at the cathode Chlorine ions are oxidized at the anode