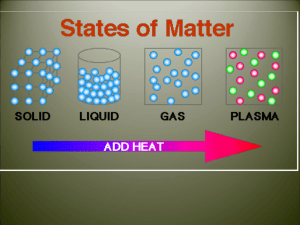
cooling curve
advertisement
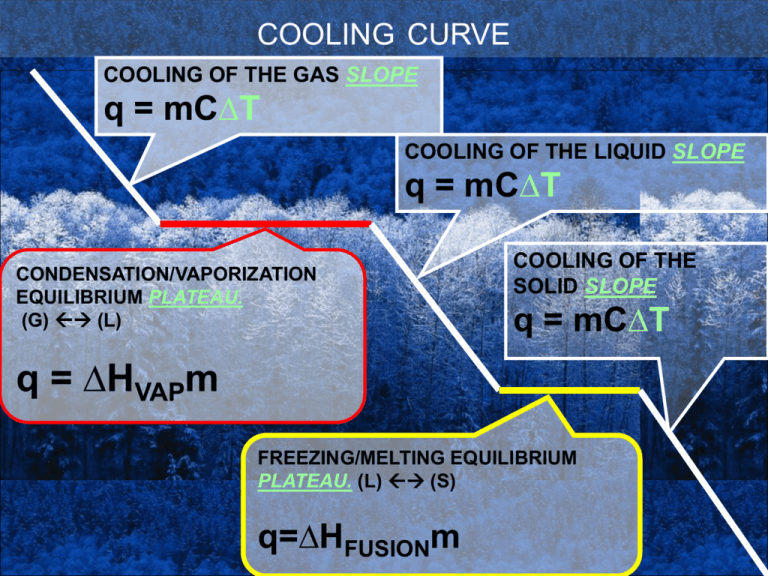
COOLING CURVE COOLING OF THE GAS SLOPE q = mC∆T COOLING OF THE LIQUID SLOPE q = mC∆T CONDENSATION/VAPORIZATION EQUILIBRIUM PLATEAU. (G) (L) COOLING OF THE SOLID SLOPE q = mC∆T q = ∆HVAPm FREEZING/MELTING EQUILIBRIUM PLATEAU. (L) (S) q=∆HFUSIONm EVAPORATION -Attractions BROKEN as gas heats. MELTING -STRONG ATTACTIONS WEAKENED. LIQUID PHASE HEATING OF LIQUID -”STICKY” attractions -POTENTIAL ENERGY CHANGE. -Assumes shape of container but not the volume, -energy is gainedendothermic. -Not compressible. -fluidity, viscosity. -EVAPORATION occurs at constant temp, POTENTIAL ENERGY CHANGE. GAS PHASE HEATING OF GAS -No attractions -Assumes both shape and volume of container, compressible. -can be heated infinitely. GAS PHASE COOLING OF GAS -No attractions -Assumes both shape and volume of container, compressible. -can be heated infinitely. CONDENSATION -Attractions form as gas cools causing condensation -Condensations occurs at constant temp, POTENTIAL ENERGY CHANGE. LIQUID PHASE COOLING OF LIQUID -”STICKY” attractions -Assumes shape of container, -Not compressible. -fluidity, viscosity. FREEZING -STRONG ATTACTIONS FORMED -POTENTIAL ENERGY CHANGE. -energy is lostexothermic. SOLID -Strong attraction s. lowest energy COOLING CURVE (a) – first appearance of liquid (c) – first appearance of solid (b) – condensation nearly complete (d) – freezing complete. • THE GAS PHASE • No attractions (ideal gas). Real gases do have attractions – proof is they condense. • Move in random straight line motion, collide with each other and container wall. • Assume shape and volume of container. • Compressible – volume is indirect with pressure and direct with temp. Variable density. • At the boiling point, the gas either condenses (cooling curve) or evaporates (heating curve.) • At the higher energy plateau (potential energy) there is an equilibrium of gas and liquid at the same temperature at the same time. And condensation and vaporization ALWAYS occur together. • THE LIQUID PHASE • Particles have attractions which are not as strong as in the solid. • Assumes shape but NOT the volume of the container. Not compressible with uniform density. • Particles have some freedom of motion that causes fluidity, however they are not independent as in the gas phase. • Viscosity or “thickness” is a function of attraction strength and temp. • Some of the particles have enough energy to break attractions and escape into the gas phase below the boiling temp. These newly vaporized particles are called VAPOR and exert vapor pressure. The vapor pressure is a direct function of pressure. • MELTING/FREEZING EQUILIBRIUM • The solid and liquid exist at equilibrium at the same time at the same temperature (freezing point/melting point). • Melting (fusion) and freezing occur together ALWAYS in an equilibrium. Freezing is faster in a cooling curve, melting is faster in a heating curve. • Attraction strengthen as a substance freezes, attractions weaken as a substance melts. • THE SOLID STATE. • Assumes neither the shape or volume of its container. • The strongest attractions of any phase. • No momentum, however particles can vibrate in place. • Solids are not compressible and have uniform density. U DO IT NOW!!! 1)CALCULATE THE q WHEN 100.0 G OF WATER GAS IS COOLED FROM 120.0 oC to 100.0 oC. 2)CALCULATE THE HEAT REMOVED WHEN 100 g OF WATER IS CONDENSED AT 100oC. 3)CALCULATE THE HEAT REMOVED WHEN 100g OF WATER LIQUID IS COOLED FROM 100.0 TO 0.0 oC. 4) CALCULATE THE HEAT REMOVED WHEN 100G OF WATER FREEZES, 5) CALCULATE THE HEAT REMOVED (q) WHEN 100G OF ICE IS COOLED FROM 0.0 C TO -20 C. • 1)CALCULATE THE q WHEN 100.0 G OF WATER GAS IS COOLED FROM 120.0 oC to 100.0 oC. q = mC∆T Q = 100.0g * 4.18 J * -20oC = - 8360 goC ∆T is negative when T decreases, as is q • 2)CALCULATE THE HEAT REMOVED WHEN 100 g OF WATER IS CONDENSED AT 100oC. q = m-Hvap Q = 100.0g * -2260.0 J = -226000.0 J g Condensation is exothermic, has a negative sign. It is the reverse of evaporation. Condensation is EXOTHERMIC, q is negative • 3)CALCULATE THE HEAT REMOVED WHEN 100g OF WATER LIQUID IS COOLED FROM 100.0 TO 0.0 oC. q = mC∆T q = 100.0g * 4.18 J * -100oC = -41800 J = 41.800kJ goC • 4) CALCULATE THE HEAT REMOVED WHEN 100G OF WATER FREEZES, q = mHfusion q = 100.0g * -334.0 J = -33400.0 J = -33.400 kJ g FREEZING Q = ∆Fusion = - MELTING Q=+ ∆Fusion = + CONDENSATION Q=∆vaporization = - VAPORIZATION Q=+ ∆vaporization= + EXOTHERMIC: Energy EXITS, is released ENDOTHERMIC Energy ENTERS, is absorbed EXOTHERMIC: Energy EXITS, is released ENDOTHERMIC: Energy ENTERS, is absorbed The heat is equal in number but opposite in sign to heat of fusion Heat of fusion is positive The heat is equal in number but opposite in sign to heat of vaporization Heat of vaporization is positive