FIRST LAW OF THERMODYNAMICS
advertisement

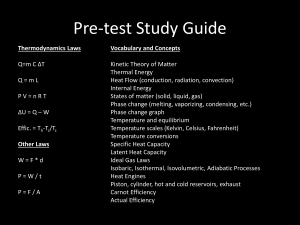
INTRODUCTION TO NAVAL ENGINEERING THERMODYNAMICS II INTRODUCTION TO NAVAL ENGINEERING FIRST AND SECOND LAWS OF THERMODYNAMICS FIRST LAW OF THERMODYNAMICS Energy can be neither created nor destroyed but only transformed THE GENERAL ENERGY EQUATION Energy In = Energy Out or U2 - U1 = Q - W where U1: internal energy of the system at the beginning U2: internal energy of the system at the end Q : net heat flow into the system W : net work done by the system ELEMENTS OF A THERMODYNAMIC CYCLE (1) Working Substance - medium by which energy is carried through the cycle. (2) Heat Source - supplies thermal energy to the working substance. (3) Heat Receiver - absorbs heat from the working substance. ELEMENTS OF A THERMODYNAMIC CYCLE (4) Pump - circulates the working substance; acts as a valve between low and high pressure (5) Engine - device which converts the thermal energy of the working substance into useful mechanical energy HEAT SOURCE ENGINE PUMP HEAT RECEIVER THERMODYNAMIC CYCLES CLOSED – Working fluid never leaves the cycle, except through accidental leakage (ex: steam cycle) OPEN – Working fluid is taken in, used, then discarded (ex: internal combustion engine) ENGINES HEATED: – heat is added to the working substance in the engine itself (ex: internal combustion engine) UNHEATED: – the working substance receives heat in some device that is separate from the engine (ex: steam turbines) HEAT SOURCE UNHEATED ENGINE PUMP HEAT RECEIVER WORKING SUBSTANCE (MIXTURE OF ATMOSPHERIC AIR AND FUEL) LOW PRESSURE OR HIGH PRESSURE SIDE OF THE CYCLE, DEPENDING UPON POSITION OF PISTON IN THE CYLINDER HEAT RECEIVER (ATMOSPHERE) HEAT SOURCE (COMPRESSION OR SPARK) PUMP (PISTON) HEATED ENGINE (PISTON AND CYLINDER) FLOW PROCESSES NON-FLOW – One in which the working fluid does not flow into or out of its container in the course of the process (ex: air compressors, internal combustion engines) STEADY FLOW – One in which a working substance flows steadily and uniformly (ex: boilers, turbines, condensers) USING THE G.E.E. (Non-Flow) Q12 = (U2 - U1) + wk12/J where Q12 U2, U1 wk12 J total heat transferred (Btu) total internal energy at points 1 and 2 (Btu) work done between states 1 and 2 (ft-lbs) constant of 778 ft-lbs/Btu WORKING SUBSTANCE WORKING SUBSTANCE PUMP (PISTON) PUMP (PISTON) G.E.E. (Non-Flow) EXAMPLE 5 LBM of a fluid is compressed in the cylinder using 350 Btus of work. If internal energy initially was 100 Btu/lbm and 150 Btu/lbm at the end of the compression, how much heat was added/lost? Q12 = (U2 - U1) + wk12/J STEADY FLOW SYSTEMS AND THE GENERAL ENERGY EQUATION ENTHALPY (H or h) COMBINATION OF: – INTERNAL ENERGY (U) – FLOW WORK • Mechanical energy necessary to maintain the steady flow of the fluid • Flow work = pV/J (Btu) H = pV/J + U STATE CHANGES Isobaric – the pressure of and on the working fluid is constant Isenthalpic – the enthalpy of the working fluid does not change (h1 = h2) Isothermal – temperature is constant Adiabatic – occurs in such a way that there is no transfer of heat to or from the system during the process THE SECOND LAW OF THERMODYNAMICS (1) All energy received as heat by a heat engine cycle cannot be converted into work (This means that no cycle can have a thermal efficiency of 100%) THE SECOND LAW OF THERMODYNAMICS (2) The transformation of heat to work is dependent on a temperature difference and on the flow of heat from a high temperature reservoir to a low temperature reservoir. (In other word heat must flow from hot to cold) THE SECOND LAW OF THERMODYNAMICS (3) It is impossible to construct an engine that, operating in a cycle, will produce no effect other than the transfer of heat from a low temperature reservoir to a high temperature reservoir THE SECOND LAW OF THERMODYNAMICS “FRICTION HAPPENS” ENTROPY A THEORETICAL MEASURE OF ENERGY THAT CANNOT BE TRANSFORMED INTO MECHANICAL WORK IN A THERMODYNAMIC SYSTEM. The total amount of entropy in a system always goes up. No thermodynamic process can occur without some losses. First Law: “You can’t win” Second Law: “You can’t even break even” ENTROPY (cont) A MEASURE OF DISORDER – always growing in our universe “THE END OF THE UNIVERSE IS UPON US” CRUCIAL PART OF REAL THERMODYNAMIC EQUATIONS CARNOT CYCLE TEMP ENTROPY CARNOT EFFICIENCY work output η heat input Tsource - Treceiver η Tsource Source (Ts) Heat Flowin = CpTs = Qin Workout = Qin - Qout Engine Carnot = Workout Ts - Tr = Qin Ts Heat Flowout = CpTr = Qout Receiver (Tr) CARNOT EFFICIENCY FOR IDEAL ENGINES Work Output