Electrolyte_Disturbances
advertisement

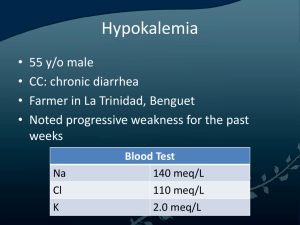
Electrolyte Disturbances Introduction • Main electrolytes in the blood – sodium, potassium, calcium, magnesium, chloride, phosphate, and carbonate • Most commonly, problems occur when the level of sodium, potassium, or calcium is abnormal • Levels can become high or low • Often, electrolyte levels change when water levels in the body change Hypokalemia • Most common electrolyte abnormality • Definition < 3.6 mmol/L – Most between 3.0 and 2.5 mmol/L • >20% hospitalized patients (No comparable data available for outpatients) • Hypokalemia has been found in 20-40% of those patients treated with diuretics Hypokalemia • Often asymptomatic and well-tolerated in otherwise healthy individuals • Can be life-threatening when severe • May the risks of morbidity and mortality in patients with cv disease, even when mild or moderate As a result, when identified it is important to determine any underlying etiologies and treat appropriately Regulation of Potassium Balance • Total body stores (intracellular and extracellular fluid) are closely regulated by key hormones – – – – Insulin β-adrenergic catecholamines Aldosterone Thyroid hormone • Normal: High ratio of intracellular to extracellular potassium • Both insulin and β- adrenergic catecholamine cellular potassium uptake by stimulating cell membrane Na+/K+-ATPase Regulation of Potassium Balance • Insulin: feedback system – ↑↑ K+ stimulates insulin – ↓↓ K+ inhibits insulin • β-adrenergic: no feedback system – β blockade ↑↑ serum K+ – β agonist ↓↓ serum K+ • Thyroid Hormone: + synthesis of Na+/K+ ATPase • Aldosterone: – ?? affects on the transcellular distribution of potassium – major regulator of body stores of potassium via its effects on the excretion of potassium by the kidney Regulation of Potassium Balance Clinical Manifestations of Hypokalemia • Often asymptomatic, usually when mild (3-3.5 mmol/L) • Nonspecific symptoms with more severe hypokalemia – generalized weakness; lassitude; constipation • Serum K+ < 2.5 mmol/L muscle necrosis • Serum K+ <2.0 mmol/L ascending paralysis and impairment of respiratory function • Symptoms usually correlate with rapidity of serum K+ Hypokalemia and Cardiac Manifestations • In patients without underlying heart disease, abnormalities in cardiac conduction is very rare, even when the serum potassium <3.0 mmol/L • risk of development of a cardiac arrhythmia (even with mild to moderate hypokalemia) – heart failure, cardiac ischemia, and/or LVH • Hypokalemia the arrhythmogenic potential of digoxin Etiology of Hypokalemia • Two main mechanisms: 1. Abnormal losses • Increased renal potassium loss • Excess potassium loss in stools 2. Transcellular potassium shift • Drugs are the most common cause Drug Induced Hypokalemia • Transcellular Potassium Shift – Β2-Adrenergic Drugs • Increase Renal Potassium Loss – Diuretics – Mineralocorticoid/Glucocorticoid Effects – Antibiotics • Excess Potassium Loss in Stool Β2-Adrenergic Drugs • Bronchodilators – a standard dose of nebulized albuterol ↓serum K by 0.2 to 0.4 mmol/L – second dose within one hour ↓ K by 1 mmol/L • Decongestants • Tocolytic Agents – ↓ serum K to as low as 2.5 mmol/L after 4-6 hours of IV administration Drug-Induced Hypokalemia Transcellular Shifts • Xanthines – Theophylline • Severe hypokalemia with acute theophylline toxicity – Caffeine • Verapamil intoxication • Chloroquin intoxication – Inhibits potassium from exiting cells • Insulin – Moves potassium into cells – Transient hypokalemia – Intentional overdose of insulin; treatment of DKA Thiazide and Loop Diuretics • Most common cause of hypokalemia • Block Cl-associated Na+ reabsorption ↑ delivery of Na+ to collecting tubules↑ Na+ reabsorption ↑ K+ secretion • Direct relation with dose • response of hypokalemia with more dietary Na+ intake Diuretics • Furosemide or Bumetanide with Metolazone Moderate-Severe Hypokalemia • Associated mild to moderate alkalosis • Acetazolamide promote K+ reabsorption – Interferes with H+ linked sodium reabsorption metabolic acidosis Drugs with Mineralocorticoid or Glucocorticoid Effects • Fludrocortisone – Oral mineralocorticoid – promotes renal K+ excretion – If used inappropriately can lead to K+ wasting • Prednisone and Hydrocortisone – Glucocorticoids – indirectly K+ secretion via effect on filtration rate and distal sodium delivery – ↓ by 0.2 to 0.4 mmol/L Other drugs causing renal wasting of potassium • High dose antibiotics – Na+ delivery to nephrons – PCN, Naficillin, Ampicillin, Carbenicillin • Drugs assoiciated with Mg depletion – Aminoglycosides – Cisplantin – Foscarnet – Amphotericin B Non-Drug Causes of Hypokalemia • Transcellular shifts • Inadequate dietary intake • Abnormal losses of potassium – Looses in stool – Loss through the kidney Non-Drug Causes Due to Transcellular Shifts • Hyperthyroidism – Severe <3.0 mmol/L sudden onset of severe muscle weakness and paralysis – 2 to 8% of patients with hyperthyroidism in Asian countries • Familial Hypokalemic Periodic Paralysis – autosomal dominant – Mutations of gene encoding the dihydropyridine receptor – Sudden attacks of muscle paralysis, K+ < 2.5 mmol/L – Provoked by high intake of CHO, sodium, or by exertion – Subside < 24 hours Non-Drug Causes Due to Transcellular Shifts • Delirium tremens – Abrupt ↓ serum K+ by 1mmol/L – Β2-adrenergic stimulation • Barium compounds – Block exit of K+ from cells – Vomiting and diarrhea – Muscle weakness, paralysis, rhabdomyolysis • Severe pernicious anemia – Treatment with vitamin B12 rapid uptake of K+ by new cells formed – Transfusion with frozen washed red cells Non-Drug Causes Due to Inadequate Dietary Intake • potassium intake by <1gram/day (25 mmol/day) • Renal excretion of potassium fails to decrease promptly • With starvation or near-starvation depletes body K+ stores compensated by tissue breakdown that releases K+ Non-Drug Causes Due to Abnormal Losses of Potassium • Stool losses • Renal losses – Metabolic Alkalosis • Chloride sensitive: vomiting, nasogastric drainage • Chloride insensitive: hyperaldosteronism, Cushing’s syndrome • Genetic abnormalities: Liddle’s syndrome, 11β-hydroxysteroid dehyrogenase deficiency, Bartter’s syndrome, Gitelmans’s syndrome – Metabolic Acidosis • Type I or classic distal renal tubular acidosis • Type II or proximal renal tubular acidosis Treatment of Hypokalemia • Potassium replacement • Note, that supplemental potassium administration is the most common cause of severe hyperkalemia in hospitalized patients • Greatest risk with IV replacement – If given, rate should be no more than 20 mmol/hour and monitor cardiac rhythm • PO administration safer because enters circulation slower Treatment of Hypokalemia • On average, serum potassium by 0.3 mmol/L for each 100 mmol reduction in total body stores • A portion of administered potassium is always excreted, even in presence of serious potassium depletion Treatment of Hypokalemia • Three salts available – Potassium chloride – Potassium phosphate – Potassium bicarbonate • KCl most often used because of its unique effectiveness with the most common cause of potassium depletion • Liquid or tablet form of KCl available • Initially, 1 mcg of KCL serum K+ by 0.1 mmol/L Treatment of Hypokalemia • Typically, 40 to 100 mmol of supplemental K+ each day to maintain wnl serum K+ with daily use of diuretics • Hypokalemia persists in patients taking diuretics despite aggressive potassium replacement in 10% of patients • May add a 2nd diuretic that inhibits K+ excretion – Amiloride, triamterene, or spironolactone Hyperkalemia • Diagnosed in up 8% of hospitalized patients • Primary cause of morbidity and death is potassium's effect on cardiac function • Mortality rate can be as high as 67% if severe hyperkalemia is not treated rapidly • Hyperkalemia is defined as a potassium level >5.5 mEq/L 5.5 - 6.0 mEq/L 6.1 - 7.0 mEq/L 7.0 mEq/L and greater Mild Moderate Severe Causes of Hyperkalemia • or impaired K+ excretion – acute or chronic renal failure (most common) – K+ sparing diuretics, urinary obstruction, sickle cell disease, Addison disease, and SLE • Additions of potassium into extracellular space – K supplements, rhabdomyolysis, hemolysis • Transmembrane shifts – acidosis and medication effects (eg, acute digitalis toxicity, BB, succinylcholine) • Factitious or pseudohyperkalemia – improper blood collection, lab error, leukocytosis, and thrombocytosis Clinical Manifestation • Hyperkalemia frequently discovered as an incidental laboratory finding • However may experience: – – – – – Generalized fatigue Weakness Paresthesias Paralysis Palpitations • EKG findings – Early: peaked T waves, shortened QT interval, and ST segment depression – Later: bundle branch block, resulting in widened QRS, increases in the PR interval, and decreased amplitude of the P wave Hyperkalemia Approach to Hyperkalemia • Is this a true measurement? – Look at prior measurements – Repeat stat • Is patient symptomatic or have underlying reason to explain for the ↑↑ K+? – Renal insufficiency – Medications (BBlockers, K+sparing diuretics, K+ supplements, digitalis) • EKG findings? Approach to Hyperkalemia • ABC’s • IV access and cardiac monitor • EKG • Discontinue any potassium-sparing drugs or dietary potassium • If the hyperkalemia is severe (>7.0 mEq/L) or the patient is symptomatic begin treatment, do not wait for tests – Individualize treatment based upon the patient's presentation, potassium level, and EKG Treatment of Hyperkalemia • Calcium gluconate – Stabilizes cell membrane – 2 amps IV; onset few minutes • Insulin – Drives K into cells – 10 units regular + 1-2 amps D5W; onset 15-30min • Bicarbonate – Drives K into cells in exchange for H – 1-3 amps IV; onset 15-30 minutes • Kayexlate – Exchanges Na for K in GI tract – 30-90 grams po/pr; onset 1-2 hours • Diuretics – Furosemide ≥ 40 mg IV; onset 30 minutes • Hemodialysis Sodium and Water Homeostasis • Disorders of sodium are generally due to changes in total body water, not body sodium • Two key hormones: – Antidiuretic hormone (ADH) • Primary hormone regulator of sodium concentration – Aldosterone • Primary hormone regulator of total body sodium (and therefore volume) Antidiuretic Hormone (ADH) • Stimuli for secretion: hyperosmolality, ↓↓ effective arterial volume (EAV) • Action: open water channels in collecting ducts passive water reabsorption into medulla • Urine osmolality- indirect assay of ADH activity – Uosm range 60 mOsm/L (no ADH activity) to 1200 mOsm/L (max ADH activity) • ↑↑ ADH = Syndrome of inappropriate ADH (SIADH) • ↓↓ ADH = Central diabetes insipidus Aldosterone • Stimuli for secretion: – hypovolemia (via renin and angiotensin II) – hyperkalemia • Action: isoosmotic reabsorption of sodium in exchange for potassium or H+ • ↑↑ aldosterone = Conn’s Syndrome ( HTN, hypokalemia, metabolic alkalosis) • ↓↓ aldosterone = hypovolemia, hyperkalemia, metabolic acidosis Hyponatremia • A common clinical problem and frequently develops in hospitalized patients • Although morbidity varies widely in severity, serious complications can arise form the disorder itself as well as from errors in management • Defined as the excess of water relative to sodium • A serum sodium concentration <136 mmol/L Approach to Hyponatremia • FIRST, determine tonicity – Isotonic • Rare laboratory artifact due to hyperlipidemia or hyperproteinemia check lipid panel and/or lft’s – Hypertonic • Excessive presence of other effective osmole – Glucose, mannitol, or glycine – For each 100 mg/dL rise in glucose above 100 mg/dL the Na ↓ 1.6 mEq/L – Hypotonic • True excess of water relative to sodium Approach to Hyponatremia • NEXT, if true excess of water relative to sodium, in other words, hypotonic hyponatremia, determine volume status – – – • Hypovolemic Euvolemic Hypervolemic Vital signs, orthostatics, JVP, skin tugor, mucous membranes, peripheral edema, BUN, Cr, uric acid Hypotonic Hyponatremia • Three categories: – Hypovolemic Hypotonic Hyponatremia – Euvolemic Hypotonic Hyponatremia – Hypervolemic Hypotonic Hyponatremia Hypovolemic Hypotonic Hyponatremia • Primary Na loss secondary H20 gain • Determine renal vs. extrarenal losses – UNa > 20 mEq/L = renal losses • Diuretics • Hypoaldosteronism • Salt-wasting nephropathy – UNa < 10 mEq/L = extrarenal losses • GI losses • Third-spacing • Insensible losses Hypervolemic Hypotonic Hyponatremia • Determine if there is a ↓ EAV or advanced renal failure • UNa < 10 mEq/L = ↓ EAV – CHF (↓ CO) – Cirrhosis (ascites) – Nephrotic Syndrome (hypoalbuminemia edema) • UNa > 20 mEq/L = advanced renal failure Euvolemic Hypotonic Hyponatremia • Primary H2O gain • Determine whether SIADH or ADH suppression – Uosm>100 = SIADH – Uosm <100 = psychogenic polydipsia • >12- 20 L/day – Uosm variable = ADH physiology reset to regulate lower serum Na concentration Etiology of SIADH • Endocrine – Hypothyroidism, Adrenal Insufficiency • Pulmonary – Pneumonia, Asthma, COPD, pneumothorax • CNS – Trauma, infection, hemorrhage, hydrocephalus, CVA, demyelinating diseases, acute psychosis • Malignancy – Small cell lung cancer, intracranial tumors • Drugs – Thiazides, antidepressants, antipsychotics • Others: Post-operative state, pain, nausea Approach to Hypotonic Hyponatremia Clinical Manifestation of Hyponatremia • Severity of symptoms related to how rapid and to what degree the hyponatremia develops • Often with Na+ >125 asymptomatic or may have nonspecific symptoms – Headache, nausea, vomiting, muscle cramps, lethargy, restlessness, disorientation, depressed reflexes • More severe or rapidly developed hyponatremia – Seizures, coma, permanent brain damage, respiratory arrest, brain-stem herniation, and possibly death Management of Hyponatremia • The optimal treatment of hypotonic hyponatremia requires balancing the risks of hypotonicity against those of therapy • The presence of symptoms and their severity largely determine the pace of correction • Too rapid correction ↑ serum osmolality in setting of low brain osmolality rapid water egress acute brain dehydration central pontine myelinosis Management of Hyponatremia • No consensus about the optimal treatment of symptomatic hyponatremia • Most reported cases of osmotic dymyelination occurred after corrections of only 9 to 10 mmol/L in 24 hours or 19 mmol/L in 48 hours • Targeted rate of correction that does not exceed 8 mmol/L on any day of treatment • The initial rate of correction can still be 1 to 2 mmol/L/hour x several hours in patients with severe symptoms Management of Hyponatremia • Hypovolemic Hyponatremia – Volume replacement, often with 0.9%NaCl • Hypervolemic Hyponatremia – Sodium (1-3 g/day) and water (1.0-1.5 L/day) restriction • SIADH – Free water restriction – If chronic demeclocycline – If neurologic emergency loop diuretic + hypertonic saline Calculating fluid replacement In cases of hypovolemic or severe SIADH with neurological manifestations . . . 1. Calculate Na+ deficit: NA deficit= 0.6 x ideal body weight x (140 – measured Na) (x 0.85 for women) 2. Choose appropriate infusate 3. Calculate # liters of chosen saline required 4. Calculate rate of infusion, keeping in mind the danger of correcting too fast and at the same time the consequences of the untreated hypotonicity CASE 1 • A 58 year-old man with small cell lung cancer presents with confusion and lethargy. Clinically euvolemic. IBW 80 kg. • LAB: Na 112, K 3.9, Cr 0.5, Serum Osm 220, Uosm 600, TSH 2.3, random serum cortisol 24 • How do you classify this patient’s hyponatremia? Euvolemic Hypotonic Hyponatremia Tumor-Induced SIADH • How do you manage this patient? CASE 1 1. Calculate Na deficit 0.6 x 80 x (120-112)= 384 mEq of Na 2. Choose infusate 3% NaCl (513 mmol/L of Na) 3. Calculate # liters of 3% NaCl required 384 mEq Na / 513 mmol/ L Na= 750 ml 4. Determine rate of infusion 750cc/24 hour= 31ml/hr Hypernatremia • Whereas, hyponatremia can be associated with low, normal, or high tonicity hypernatremia always denotes hypertonicity • Deficit of water relative to sodium • Almost always due to loss of hypotonic fluid and impaired access to free water • Hypernatremia is a powerful thirst stimulus Approach to Hypernatremia • Determine volume status – Hypovolemic – Euvolemic – Hypervolemic Approach to Hypernatremia • Determine volume status – Hypovolemic, euvolemic water loss check Uosm • Uosm <300 • Uosm 300-600 • Uosm >600 complete DI renal H20 losses, partial DI, reset osmostat extrarenal H2O losses – Hypervolemic Na retention • Exogenous NaCl infusion • Mineralocorticoid excess H20 loss and Hypernatremia • Diabetes insipidus – Central • CNS trauma, surgery, hemorrhage, infection, granulomas, tumor, idiopathic – Nephrogenic • Drugs: lithium, amphotericin, demeclocycline • Metabolic: hypercalcemia, severe hypokalemia • Others: polycystic kidney disease, sickle cell, Sjogren’s syndrome, sarcoid, amyloid • Renal losses: diuretics, osmotic diuresis (glucose, urea, mannitol) • Extra-renal losses: GI or insensible losses Treatment of Hypernatremia • Avoid rapid correction – ↓ serum osmolality in setting of high brain osmolality water ingress cerebral edema • Hypovolemic Hypernatremia – Replace volume & free water deficit – Free water deficit= 0.6 x ideal body weight x [(measured Na/140)-1] (x 0.85 for female) • Hypervolemic Hypernatremia – Loop diuretic + D5W • DI – Central DI: desmopressin (dDAVP) – Nephrogenic DI: treat underlying cause; salt restriction + thiazide diuretics Calcium Homeostasis • 40% of the calcium is bound to protein • 50% is ionized and is in physiologic active form • Remaining 10% complexed to anions • Corrected total calcium (mg/dL) = (measured total calcium mg/dL) + 0.8 (4.4 measured albumin g/dL) Calcium Homeostasis • Three major hormones involved in calcium homeostasis – parathyroid hormone (PTH) – 1,25-dihydroxyvitamin D (calcitriol) – Calcitonin • PTH directly targets the bone and the kidneys to serum calcium levels. Indirectly, through vitamin D, it causes intestinal calcium absorption • Vitamin D directly targets GI absorption of calcium to increase calcium levels • Calcitonin lowers calcium by targeting bone, renal, and GI losses Hypercalcemia • Primary hyperparathyroidism – most common cause – occurs in 25 per 100,000 persons in the general population and in 75 per 100,000 hospitalized patients • Hypercalcemia has been reported to occur in up to 20 to 30% of patients with cancer • The detection of hypercalcemia in a patient with cancer signifies a very poor prognosis; approximately 50% of such patients die within 30 days Causes Of Hypercalcemia • Hyperparathyroidism – Primary: adenoma (80%), hyperplasia (15-20%), carcinoma (<1%) – Secondary: response to hypocalcemia – Tertiary: long standing secondary autonomous nodule develops • Malignancy – Local osteolytic (breast, melanoma) – Solid tumor secreting PTH-related peptide (SC lung Ca, RCC) – Hematologic via ↑1,25-D and cytokines (Bcell lymphomas) Causes Of Hypercalcemia • Vitamin D excess (↑1,25-D) – Granulomatous diseases (TB, sarcoidosis, histo, cocci, crypto) – Vitamin D intoxication • ↑ bone turnover Hyperthyroidism, immobilization, Paget’s disease • Others: Thiazides, Vitamin A, Milk-Alkali Syndrome Clinical Manifestations of Hypercalcemia • “Bones, stones, abdominal groans, and psychic moans” usually when Ca>12 – – – – – – – Osteopenia and osteitis firbrosa cystica Neprholithiasis, nephrocalcinosis, nephrogenic DI Abdominal pain, N/V/C, anorexia Pancreatitis, PUD Fatigue, depression, confusion Decreased DTRs Short QT intervals • Hypercalcemia crisis (Ca 13-15) – Polyuria, dehydration, mental status changes Treatment of Hypercalcemia • Aggressive IVF – NS @ 200-500 cc/hour, depending on the patient’s cardiovascular status and renal function • Aggressive calciuresis with loop diuretics, after normvolemia has been restored • IV bisphosphonates to inhibit bone reabsorption – Pamidronate 60 to 90 mg IV over 2 hours – Zoledronate 4mg IV over 15 minutes • Treat underlying disease process Hypocalcemia • Probably more common than hypercalcemia • Reported in 15-50% ICU patients • Severe, symptomatic hypocalcemia may result in cardiovascular collapse, hypotension unresponsive to fluids and vasopressors, and dysrhythmias • Clinically evident hypocalcemia generally presents in milder forms and is usually the result of a chronic disease state Etiologies of Hypocalcemia • Hypoparathryoidism – PGA type I (chronic mucocutaneous candidiasis +hypothyroid + Addison’s) – s/p thyroidectomy, hypomagnesiumia • Pseudo-hypoparathyroidism – PTH end-organ resistance; normal Ca – Skeletal abnormalities & retardation • Vitamin D deficiency • Renal Failure – ↓ 1,25- (OH)2D3 production + ↑ PO4 ↑calcium deposition in soft tissue • Others – Pancreatitis, citrate excess (multiple transfusions) Clinical Manisfestations of Hypocalcemia • Neuromuscular irritability – Chvostek sign • tapping facial nerve contraction of facial muscles – Trousseau sign • inflation of a blood pressure carpal spasm – Perioral paresthesias, cramps, laryngospasm • Irritability, depression, psychosis, ↑ICP, seizures • EKG: ↑ QT • Renal osteodystrophy – ↓ vit D & ↑ PTH in renal failure osteomalacia (↓ bone mineralization), osteitis fibrosa cystica, osteoporosis Treatment of Hypocalcemia • Symptomatic IV Ca gluconate • Asymptomatic oral Ca and vitamin D • In renal failure calcitriol (1,25- (OH)2D3) • In hypoparathyroidism PTH supplementation not available, therefore give calcitriol Magnesium • 4th most common cation in the body • A central electrolyte in a large number of cellular metabolic reactions – – – – DNA and protein synthesis Neurotransmission Hormone-receptor binding A component of GTPase and a cofactor for Na+/K+– ATPase, adenylate cyclase, and phosphofructokinase – Necessary for the production of parathyroid hormone • Absorbed in the small bowel, mainly in the ileum • Excreted in stool and urine, but regulation of serum magnesium is under renal control Hypomagnesemia • Etiology – GI losses • Malabsorption of magnesium in the ileum results in GI secretions in large amounts – Malnutrition – Renal losses • Primary renal disorders • Drugs (diuretics, cisplantin) • Endocrine disorders (primary aldosteronism, hypoparathyroidism Hypomagnesemia • Symptoms are nonspecific – <1.8 mEq/L (reference range 1.8-3 mEq/L) – Weakness, muscle cramping, or rapid heartbeats; AMS – Less severe cases may result in vertigo, ataxia, depression, and seizure activity • Primary clinical findings are neuromuscular irritability, CNS hyperexcitability, and cardiac arrhythmias • Treatment – Oral for mild; IV for severe – magnesium oxide, magnesium hydroxide, magnesium gluconate Phosphate • A ubiquitous intracellular anion that is essential for membrane structure, energy storage and transport in all cells – ATP production – Red blood cells for production of 2,3diphosphoglycerate (2,3-DPG) • Normal range: 2.5-5 mg/dL • Hypophosphatemia – mild (2-2.5 mg/dL) – moderate (1-2 mg/dL) – severe (<1 mg/dL or 0.3 mmol/L). Hypophosphatemia • Etiology – – – – – Alcohol withdrawal During treatment of DKA Chronic alcoholism Chronic ingestion of phosphate-binding antacids TPN with inadequate phosphate supplementation • Symptoms – Weakness- most common – Neurologic symptoms vary; ranging from simple paresthesias to profound alterations in mental status Hypophosphatemia • Treatment – Mild to moderately severe and asymptomatic • may require oral replacement • correcting factors that led to the hypophosphatemia usually is sufficient – Minimal symptoms or moderate hypophosphatemia (serum phosphate 1-2 mg/dL) • po phosphate replacement may be desirable – Symptomatic or with serum phosphate levels <1mg/dL • require IV phosphate replacement Hyperphosphatemia • > 5 mg/dL in adults or 7 mg/dL in children or adolescents • Phosphorus load (from GI absorption, exogenous administration, or cellular release) exceeds renal excretion and tissue uptake • Majority are ESRD • ~250,000 persons are affected • Muscle cramping secondary to low calcium levels- most common complaint; may progress to tetany, delirium, and seizures Hyperphosphatemia • Etiology – Renal insufficiency (acute or chronic) – catabolism or cellular injury (rhabdomyolysis ,trauma, shock, exhaustive exercise, prolonged immobilization, massive hemolysis, severe infections) – Endocrinopathies (hypoparathyroidism, acromegaly, thyrotoxicosis) – Poisioning or excessive intake or administration (bisphosphonate, oral/rectal laxatives, enemas, hyperalimentation, transfusion of outdated blood) – Neoplasms (leukemia, lymphoma, bone tumors, tumor lysis after chemotherapy) Hyperphosphatemia • Treatment – Oral phosphate binders ( the highly efficient GI absorption of phosphorus) – Calcium salts are widely used but produce hypercalcemia – Aluminum salts are effective binders but induce aluminum toxicity – Newer compounds containing iron or bile acid sequestrants are replacing calcium and aluminum binders Remember . . . • K of 4 • Phosph of 3 • Mg of 2 References • Adrogue HJ, Madias NE. Hyponatremia. NEJM 2000;342:1581-89. • Gennari JF. Hypokalemia. NEJM 1998;339:45158. • Harrison et al. Principles of Internal Medicine 2001;15:271-83. • Stewart AF. Hypercalcemia Associated with Cancer 2005;352:373-79. • Uptodate • Pocket Medicine