phase changes
advertisement
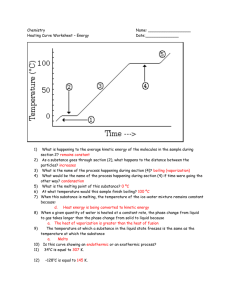
Changes of State also known as Phase Changes What are phase changes? • Phase changes (changes of state) are the processes of changing from one state of matter to another. (Example: solid to liquid) • Phase changes are physical changes, so the identity of the matter does not change. • Phase changes are processes, NOT the same things as the states of matter! • States of matter are solid, liquid, gas, and plasma. These are physical properties of matter. What causes a change of state? • Changes of state occur by adding or removing heat energy (kinetic) from matter. • Changes of state caused by adding heat energy to matter are classified as endothermic processes. (Endo = enter, heat goes in.) • Changes of state caused by removing heat energy are classified as exothermic processes. (Exo = exit, heat goes out.) • Kinetic Energy is related to the motion of the particles. If energy is added, the particles move faster. • Temperature- measure of the speed of a substance’s particles, and is the measure of the amount of thermal energy. • Heat- The transfer of thermal energy There are 6 main changes of state (phase changes) that you will need to know: MELTING • Melting is an endothermic (absorbs energy) process in which a solid changes to a liquid. • Melting point- Temperature at which a substance changes from a solid to a liquid FREEZING • Freezing is an exothermic process in which a liquid changes to a solid. VAPORIZATION • Vaporization is an endothermic process in which a liquid is changed to a gas. • This can happen in two ways: • Boiling is vaporization that occurs throughout the liquid. The temperature at which this occurs is the boiling point. • Evaporation is vaporization that occurs at the surface of a liquid. This occurs at temperatures below the boiling point. CONDENSATION • Condensation is an exothermic process in which a gas is changed to a liquid. SUBLIMATION • Sublimation is an endothermic process in which a solid is changed to a gas without first melting to a liquid. DEPOSITION • Deposition is an exothermic process in which a gas is changed into a solid, skipping the liquid phase. STATES OF MATTER SOLID Tightly packed, in a regular pattern Vibrate, but do not move from place to place LIQUID Close together with no regular arrangement. Vibrate, move about, and slide past each other GAS Well separated with no regular arrangement. Vibrate and move freely at high speeds PLASMA Has no definite volume or shape and is composed of electrical charged particles Temperature Change versus Change of State When the temperature of a substance changes, the speed of the particles also changes. When a substance is going through a change of state, its temperature does not change until the change of state is complete Temperature at which phase changes occur are unique to the type of matter (characteristic property) Heating Curve A heating curve • shows changes of state as a solid is heated. • sloped lines show increase in temperature. • plateaus (flat lines) indicate a change of state. 12 Cooling Curve A cooling curve • shows changes of state as a gas is cooled. • sloped lines indicate a decrease in temperature. • plateaus (flat lines) indicate a change of state. 13 Different matter, different temperatures for phase changes ABSOLUTE ZERO • A temperature of 0 Kelvin is called absolute zero. • It is the temperature at which scientists believe gas molecules have no kinetic energy and all motion stops. • On the Celsius scale, absolute zero is equal to -273°C. • Scientists have not yet achieved absolute zero with matter.