Heating and Cooling Curves
advertisement
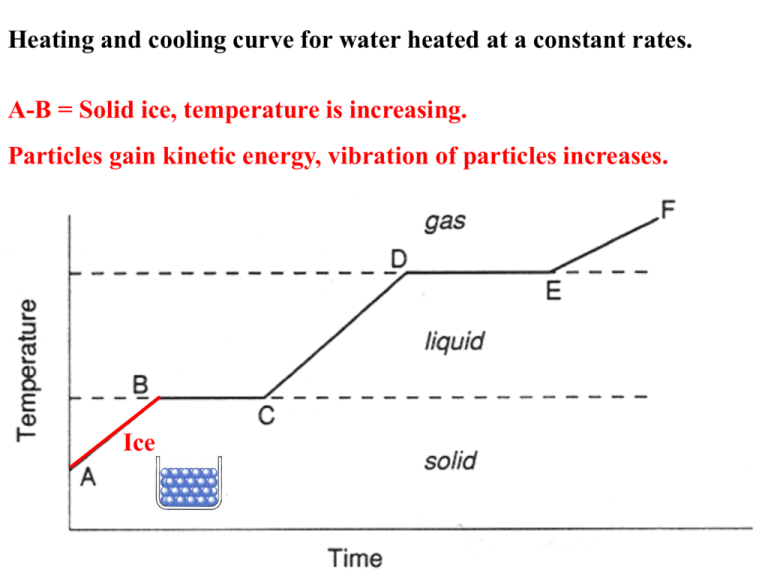
Heating and cooling curve for water heated at a constant rates. A-B = Solid ice, temperature is increasing. Particles gain kinetic energy, vibration of particles increases. Ice B-C = Solid starts to change state from solid to liquid. Temperature remains constant as energy is used to break intermolecular bonds. H2O (s) H2O () energy required 6 kJ/mol 0ºC C-D = temperature starts to rise once all the solid has melted. Particles gain kinetic energy. Liquid water D-E = Liquid starts to vaporize, turning from liquid to gas. The temperature remains constant as energy is used to break intermolecular forces. H2O () H2O (g) energy required 41 kJ/mol 100ºC E-F = temperature starts to rise once all liquid is vaporized. Gas particles gain kinetic energy. steam Heating and Cooling Curve of Water vaporization condensation freezing Endothermic and Exothermic Processes Sublimation and Deposition • Sublimation is when a solid phase goes directly into a gaseous phase without first becoming a liquid. • Deposition is when a gaseous phase goes directly to the solid phase without first becoming a liquid. Sublimation and Deposition Phase Changes Energy Changes Accompanying Phase Changes – – – – – – Sublimation: Hsub > 0 (endothermic). Vaporization: Hvap > 0 (endothermic). Melting or Fusion: Hfus > 0 (endothermic). Deposition: Hdep < 0 (exothermic). Condensation: Hcon < 0 (exothermic). Freezing: Hfre < 0 (exothermic). • Generally heat of fusion (enthalpy of fusion) is less than heat of vaporization: – it takes more energy to completely separate molecules, Ice molecules are locked in fixed positions, held by intermolecular-bonds. Ice is less dense than liquid water because the molecules are further apart than in liquid water. Energy Requirements for changing state: In ice the water molecules are held together by strong intermolecular forces. The energy required to melt 1 mole of a substance is called the molar heat of fusion (fus H) For ice it is 6.02 kJmol-1 The energy required to change 1 mole of a liquid to its vapor is called the molar heat of vaporization (vap H) For water it is 40.6 kJmol-1 H (delta H) is the change in energy or heat content. It takes more energy to vaporize water than to melt it. This is because in melting you weaken the intermolecular forces. Here about 1/6 of the hydrogen bonds are broken. In vaporization you totally break them. All the hydrogen bonds are broken vap H is always greater than fus H. Fusion is when a solid melts to form a liquid Vaporization is when a liquid evaporates to form a gas. Calculating Energy Changes: Solid to liquid How much energy is required to melt 8.5 g of ice at 0C? The molar heat of fusion for ice is 6.02 kJmol-1 Step 1: How many moles of ice do we have? n = m/M n = 8.5g / 18gmol-1 = 0.47 mol H2O Step 2: Use the equivalence statement to work the energy (6.02 kJ is required for 1 mol H2O) kJ = 0.47 mol H2O 6.02 kJ / mol H2O = 2.8kJ What is specific heat capacity? The amount of energy required to change the temperature of one gram of a substance by 1C . 10 C 11 C Another name for specific heat is a calorie (1 calorie = 4.184 Joules) Specific heat capacity of liquid water (H2O (L) ) is 4.18 J g-1C–1. Water (s) = 2.03 J g-1 C –1 Water (g) = 2.0 J g-1 C –1 0.5 cal/g to break up ice Calculating the energy to increase the temperature of liquid water. Calculating specific heat using the equation: Q = ms (tf ti) or Q = energy (heat) required Q = ms T or s = specific heat capacity Heat (H) = ms (tf ti) m = mass of the sample T = change in temperature in C EXAMPLE: How much energy does it take to heat 10g of water from 50 to 100 C ? Specific heat capacity of water = 4.184 J g-1C–1 Q = m s T Q= (10g) (4.184 J g-1 C -1) (50 C) = 2.1 10 3 J Problem How much energy is required to heat 25 g of liquid water from 25C to 100C and change it to steam? Step 1: Calculate the energy needed to heat the water from 25C to 100C Q = m s T Q = 25g 4.184 J g-1 C -1 75 C = 7.8 10 3 J Step 2: Vaporization: Use the vap H to calculate the energy required to vaporize 25g of water at 100C Molar heat of vaporization of water is 40.6 kJmolThe vap H is per mole, not per gram. So first you have to convert the grams into moles. .25g 1mol H2O / 18g mol-1 H2O = 1.4 mol H2O vap H (H2O) = 1.4 mol H2O 40.6kJ/mol = 57 kJ n(H2O) = mass / molar mass = 25g / 18g mol-1 = 1.4 mol H2O vap H (H2O) = 40.6kJ/mol vap H (H2O) = 1.4 mol 40.6kJ/mol = 57 kJ Total energy change is: 7.8kJ + 57kJ = 65kJ







