Transformation
advertisement

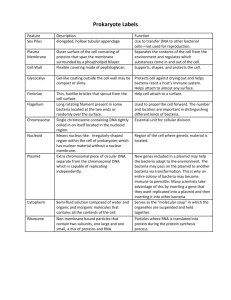
Transferencia del material genético. Conjugación, transformación y transducción. Mapeo genético Mutations in Bacteria • Mutations arise in bacterial populations – Induced – Spontaneous • Rare mutations are expressed – Bacteria are haploid – Rapid growth rate • Selective advantage enriches for mutants • Gene transfer occurs in bacteria Gene Mapping in Bacteria and Bacteriophages Mapping bacteria, 3 different methods: Conjugation Transformation Transduction Bacteriophage mapping: Bacteriophage gene mapping Cis-trans complementation test Bacteria transfer (or receive) genetic material 3 different ways Transfer always is unidirectional, and no complete diploid stage forms. 1. Conjugation 2. Transformation 3. Transduction Mating types in bacteria – Donor • F factor (Fertility factor) – F (sex) pilus – Recipient Donor • Lacks an F factor Recipient General Features of Gene Transfer in Bacteria • Unidirectional – Donor to recipient • Donor does not give an entire chromosome – Merozygotes • Gene transfer can occur between species Conjugation 1. Discovered by Joshua Lederberg and Edward Tatum in 1946. 2. Unidirectional transfer of genetic material between donor and recipient cells by direct contact. 3. Segment (rarely all) of the donor’s chromosome recombines with the homologous recipient chromosome. 4. Recipients containing donor DNA are called transconjugants. Lederberg & Tatum (1946) Experiment demonstrating recombination in E. coli. • Recombination of 2 complimentary auxotrophs gives rise to a strain that can synthesize all nutrients. Bernard Davis experiment demonstrated that physical contact is required for bacterial recombination. E. coli conjugation Conjugation-transfer of the sex factor F 1. William Hayes (1953) demonstrated that genetic exchange in E. coli occurs in only one direction. 2. Genetic transfer is mediated by sex factor F. 3. Donor is F+ and recipient is F-. 4. F is a self-replicating, circular DNA plasmid (1/40 the size of the main chromosome). Conjugation-transfer of the sex factor F 5. F plasmid contains an origin sequence (O), which initiates DNA transfer. Also contains genes for hair-like cell surface (F-pili or sexpili), which aid in contact between cells. 6. No conjugation can occur between cells of the same mating type. 7. Conjugation begins when the F plasmid is nicked at the origin, and a single strand is transferred using the rolling circle mechanism. 8. When transfer is complete, both cells are F+ double-stranded. Transfer of the F factor Conjugation of highfrequency recombinant strains 1. No chromosomal DNA is transferred by standard sex factor F. 2. Transfer of chromosome DNA is facilitated by special strains of F+ integrated into the bacteria chromosome by crossing over. 3. Hfr strains = high frequency recombination strains. 4. Discovered by William Hayes and Luca Cavalli-Sforza. 5. Hfr strains replicate F factor as part of their main chromosome. Conjugation of highfrequency recombinant strains 5. Conjugation in Hfr strains begins when F+ is nicked at the origin, and F+ and bacteria chromosomal DNA are transferred using the rolling circle mechanism. 6. Complete F+ sequence (or complete chromosomal DNA) is rarely transferred (1/10,000) because bacteria separate randomly before DNA synthesis completes. 7. Recombinants are produced by crossover of the recipient chromosome and donor DNA containing F+. Transfer of the Hfr F+ factor Excision of the F+ factor also occurs spontaneously at low frequency. 1. Begin with Hfr cell containing F+. 2. Small section of host chromosome also may be excised, creating an F’ plasmid. 3. F’ plasmid is named for the gene it carries, e.g., F’ (lac) Using conjugation to map bacterial genes 1. Begin with appropriate Hfr strains selected from F+ x F- crosses and perform an interrupted mating experiment. 2. HfrH F- thr+ leu+ aziR tonR lac+ gal+ strR thr leu aziS tons lac gal strS 3. Mix 2 cell types in medium at 37°C. Using conjugation to map bacterial genes 4. Remove at experimental time points and agitate to separate conjugating pairs. 5. Analyze recombinants with selective media. 6. Order in which genes are transferred reflects linear sequence on chromosomes and time in media. 7. Frequency of recombinants declines as donor gene enters recipient later. Interrupted mating experiment Genetic map-results of interrupted E. coli mating experiment. Generating a map for all of E. coli 1. Location and orientation of the Hfr F+ in the circular chromosome varies from strain to strain. 2. Overlap in transfer maps from different strains allow generation of a complete chromosomal map. Circular genetic map of E. coli Total map units = 100 minutes ~time required for E. coli chromosome to replicate at 37°C. Significance • Gram - bacteria – Antibiotic resistance – Rapid spread • Gram + bacteria – Production of adhesive material by donor cells Transformation • Unidirectional transfer of extracellular DNA into cells, resulting in a phenotypic change in the recipient. • First discovered by Frederick Griffith (1928). • DNA from a donor bacteria is extracted and purified, broken into fragments, and added to a recipient strain. • Donor and recipient have different phenotypes and genotypes. • If recombination occurs, new recombinant phenotypes appear. More about transformation • Bacteria vary in their ability to take up DNA. • Bacteria such as Bacillus subtilis take up DNA naturally. • Other strains are engineered (i.e., competent cells). • Competent cells are electroporated or treated chemically to induce E. coli to take up extracellular DNA. Bacteria known to be capable of transformation • Natural transformation – Gram positive bacteria • Streptococcus pneumoniae, S. sanguis, B. Subtilis, B. Cereus, B. Stearothermophilus – Gram negative bacteria • Neisseria gnonorrheae, Acinetobacter calcoaceticus, Moraxella osloensis, M. urethans • Psychrobacter sp., Azotobacter agilis, Haemophilus influenzae, H. Parainfluenzae, Pseudomonas stutzeri • Artificial transformation • Escherichia coli, Salmonella thyphimurium, Pseudomonas aeruginosas y muchas otras. Transformation • Steps – Uptake of DNA • Gram + • Gram - – Recombination • Legitimate, homologous or general • recA, recB and recC genes • Significance Phase variation in Neiseseria – Recombinant DNA technology – Transformation of Bacillus subtilis Heteroduplex DNA Chemical competence • • In some bacteria, including E. coli, treatment of cells with divalent cations at low temperature, facilitates the uptake of plasmid DNA into the cell (linear DNA can be taken up, but is shredded by cytoplasmic DNases before it can do anything) Remains unclear how this works Uptake channels made of polyP, PHB, and Ca Hanahan and Bloom, 1996, Chapter 132, Escherichia coli and Salmonella, ASM Press Electroporation High field strengths result in very transient holes in the cellular envelope Under the appropriate conditions, DNA leaks in and DNA leaks out. A high concentration of plasmid outside results in a rapid influx of plasmids into the cell. Electroporation cuvette Cells go here High voltage shock How well has your transformation worked? Transformation efficiency Saturating cells (# of transformants/mg of DNA) 106-109/mg of pBR322 app. 1011 plasmids/mg pBR322 can also be analyzed as % of cells that receive plasmid Saturating DNA % of DNA molecules that successfully transform cells Protocol Sat. cells Sat. DNA Chemical 1% 12% Electro 10% 90% Natural transformation in Gram positives Examples: Streptococcus pneumoniae Bacillus subtilis • no base specificity • limited # of uptake sites (30-75) • nicked internally • complement is degraded during transport • recombines in recipient Dubnau. 1999. Ann. Rev. Microbiol. 53:217 Natural transformation in Gram negatives Examples: Haemophilus influenzae Neisseriae gonorrhoeae • sequence specific – uptake sequences • 4-8 sites/cell • no cell bound intermediate • import of ds DNA to periplasm • complement is degraded during transport into cytoplasm • recombines in recipient Dubnau. 1999. Ann. Rev. Microbiol. 53:217 Gram positive uptake machinery -dedicated machinery for the transport of DNA into the cell the reverse of a conjugal transfer system - some components similar to Tra functions Dubnau. 1999. Ann. Rev. Microbiol. 53:217 Gram-negative uptake machinary -dedicated machinery for the transport of DNA into the cell - must cross periplasm and outer membrane Dubnau. 1999. Ann. Rev. Microbiol. 53:217 Energy for driving the process? • Intracellular ATP hydrolysis • pH gradient – PMF? • Complement degradation Function for natural transformation • Nutrition • DNA repair • Genetic diversification Diferencias entre los sistemas de transformaci—nnatural codificados por Streptococcus pneumoniae y Haemophilus influenzae. Propiedad Streptococcu s Haemophilus Factores de competencia desencadenan la competencia S’ No Forma en que el DNA entr a en la cЋlula Hebra sencilla Hebra doble Fuente de DNA que puede entrar a la cЋlula Cualquiera S—lo hom—loga Forma del DNA unido a l a superficie celular Hebra doble Hebra doble Estado f’sico del DNA dent ro de la cЋlula Unido a prote’na s Contenido en el transformasoma Mapping using transformation Recombination frequencies are used to infer gene order. p+q+ o+ x p q o 1. If p+ and q+ frequently cotransform, order is p-q-o. 2. If p+ and o+ frequently cotransform, order is p-o-q. Transduction 1. Bacteriophages (bacterial viruses) transfer genes to bacteria (e.g., T2, T4, T5, T6, T7, and ). 1. Generalized transduction transfers any gene. 1. Specialized transduction transfers specific genes. 2. Phages typically carry small amounts of DNA, ~1% of the host chromosome. 3. Viral DNA undergoes recombination with homologous host chromosome DNA. Transduction • Genetic exchange mediated by bacterial viruses (bacteriophage) • Two basic types of bacterial viruses • Lytic viruses – infect cells, multiply rapidly, lyse cells • Lysogenic viruses – infect cells, can integrate into genome and go dormant (a prophage) • - at some point, can excise, multiply and lyse cells. Phage Composition and Structure • Composition – Nucleic acid Head/Capsid • Genome size • Modified bases – Protein • Protection • Infection • Structure (T4) – Size – Head or capsid Contractile Sheath Tail Tail Fibers Base Plate Infection of Host Cells by Phages • Adsorption – LPS for T4 • • • • Irreversible attachment Sheath Contraction Nucleic acid injection DNA uptake Types of Bacteriophage • Lytic or virulent – Phage that multiply within the host cell, lyse the cell and release progeny phage (e.g. T4) • Lysogenic or temperate phage: Phage that can either multiply via the lytic cycle or enter a quiescent state in the bacterial cell. (e.g., ) – Expression of most phage genes repressed – Prophage – Lysogen Bacteriophage have a range of morphologies from simple filaments to large complex structures • May contain either RNA or DNA associated with a protein coat • Almost all bacteria have phage associated with them Brock Biology of Microorganisms, vol. 9, Chapter 8 Transfer their nucleic acid into the host cell Attach to specific receptors on the surface of their host bacteria Smithsonian (Oct 2000) T4 bacteriophage on the surface of an E. coli cell Life cycle of phage Generalized transduction of E. coli by phage P1 Transduction mapping is similar to transformation mapping Gene order is determined by frequency of recombinants. If recombination rate is high, genes are close together. If recombination rate is low, genes are far apart. Mapping genes of bacteriophages 1. Infect bacteria with phages of different genotypes using two-, three-, or four-gene crosses crossover. 2. Count recombinant phage phenotypes by determining differences in cleared areas (no bacteria growth) on a bacterial lawn. 3. Different phage genes induce different types of clearing (small/large clearings with fuzzy/distinct borders). Fine structure gene-mapping of bacteriophages Same principles of intergenic mapping also can be used to map mutation sites within the same gene, intragenic mapping. 1. First evidence that the gene is sub-divisible came from C. P. Oliver ‘s (~1940) work on Drosophila. 2. Seymour Benzer’s (1950-60s) study of the rII region of bacteriophage T4. Seymour Benzer’s (1950-60s) study of the rII region of T4 1. Studied 60 independently isolated rII mutants crossed in all possible combinations. 2. Began with two types of traits: plaque morphology and host range property. 1. Growth in permissive host E. coli B; all four phage types grow. 1. Growth in non-permissive host E. coli K12(); rare r+ recombinants grow (rare because the mutations are close to each other and crossover is infrequent). Seymour Benzer’s (1950-60s) study of the rII region of T4 3. Benzer also studied 3000 rII mutants showing nucleotide deletions at different levels of subdivision (nested analyses). 4. Was able to map to T4 to level equivalent to 3 bp. 5. Ultimately determined that the rII region is subdivisible into >300 mutable sites by series of nested analyses and comparisons. Benzer’s method for identifying recombinants of two rII mutants of T4. Benzer’s map of the rII region generated from crosses of 60 different mutant T4 strains. Benzer’s deletion analysis of the rII region of T4 No recombinants can be produced if mutant strain lacks the region containing the mutation. Benzer’s deletion map divided the rII region into 47 segments. Benzer’s composite map of the rII region indicating >300 mutable sites on two different genes. Small squares indicate point mutations mapping to a given site. Seymour Benzer’s cis-trans complementation test 1. Used to determine the number of functional units (genes) defined by a given set of mutations, and whether two mutations occur on the same unit or different units. 2. If two mutants carrying a mutation of different genes combine to create a wild type function, two mutations compliment. 3. If two mutants carrying a mutation of the same gene create a mutant phenotype, mutations do not compliment. Seymour Benzer’s cis-trans complementation test. Example of complementation in Drosophila Transposable Genetic Elements • Definition: Segments of DNA that are able to move from one location to another • Properties – “Random” movement – Not capable of self replication – Transposition mediated by site-specific recombination • Transposase – Transposition may be accompanied by duplication Types of Transposable Genetic Elements • Insertion sequences (IS) – Definition: Elements that carry no other genes except those involved in transposition – Nomenclature - IS1 – Structure – Importance GFEDCBA ABCDEFG Transposase – Mutation – Plasmid insertion – Phase variation Phase Variation in Salmonella H Antigens H1 gene H1 flagella IS H2 gene H2 flagella Types of Transposable Genetic Elements • Transposons (Tn) – Definition: Elements that carry other genes except those involved in transposition – Nomenclature - Tn10 – Structure • Composite Tns – Importance • Antibiotic resistance IS Resistance Gene(s) IS IS Resistance Gene(s) IS Plasmids • Definition: Extrachromosomal genetic elements that are capable of autonomous replication (replicon) • Episome - a plasmid that can integrate into the chromosome Classification of Plasmids • Transfer properties – Conjugative – Nonconjugative • Phenotypic effects – Fertility – Bacteriocinogenic plasmid – Resistance plasmid (R factors) Structure of R Factors • RTF RTF – Conjugative plasmid – Transfer genes • R determinant – Resistance genes – Transposons R determinant