Chemistry 20
advertisement

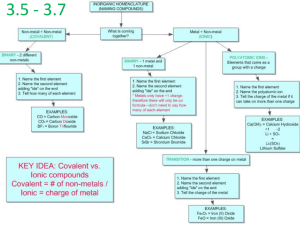
Unit 3 Part 5 Nomenclature Nomenclature IUPAC Naming System (Nomenclature) IUPAC = __ __ __ __ __ __ __ __ __ __ __ __ __ __ __ __ __ __ __ __ __ __ abcdefghijklmnopqrstuvqxyz __ __ __ __ __ __ __ __ __ __ __ __ __ __ Nomenclature (Ionic) For compounds that do not contain polyatomic ions: We first write the metal, then the non metal with “ide” added on. Ex. Li2S= AlCl3= MgO= Nomenclature (Ionic) When naming ionic inorganic compounds we always write the metal first (or the cation) and then the nonmetal (or the anion). Nomenclature (Ionic) For compounds that do contain polyatomic ions: We write the metal then the polyatomic ion (do not add the “ide” ending) Ex. K3PO4= (NH4)2CO3= Na2SiO3= MgSO4 = Nomenclature (Ionic) Transition Metals When the metal involved in the ionic bond has more than one oxidations state/possible charge we need to identify which ion it is. This usually occurs with the transition metals. Any ion that appears on your common ion sheet more than once is one of these types of metals. There are 2 ways to do this. Nomenclature (Ionic) The first way (the way that is the newer and preferred method) is to use roman numerals to identify which ion it is. Nomenclature (Ionic) Ex. Fe(CN)3= First we need to find what charge iron has (Since cyanide has -1 charge and there are 3 of them, iron must have a 3+ charge). We would then use the roman numeral III to identify this ion. The rules are the same as above but now we add the roman numeral in the middle. The compound Fe(CN)3 would be named: Nomenclature (Ionic) The second way to identify the ion is to use the -ous or -ic endings. The ion with the lower oxidation state (or lower numerical charge ignoring the + or -) gets the –ous ending, and the ion with the higher oxidation state gets the –ic ending. So in the example above, Iron 3+ is the iron ion with the higher oxidation state (the other ion is iron (II) ) so it would get the _____ending. Notice that on your table of common ions, Iron (III) also has the name _______. So Fe(CN)3 would be named ferric cyanide. Nomenclature (Ionic) (See Naming Ionic Compounds Assign Pg. 44) Writing compounds (from names) Just work backwards (and Use Criss Cross): Ex. Magnesium Chlorite Ex. Lead (II) Iodide Naming Covalent Compounds Binary compounds: These are compounds that contain only ___ elements. When naming binary COVALENT compounds we use prefixes to indicate the amount of each element present. 1 2 mono di 3 4 5 6 7 8 9 10 tri tetra penta hexa hepta octa nona deca Naming Covalent Compounds We rarely use mono, because normally we just assume that there is one if no prefix is present. Mono is only used for oxygen. Examples CO2 P4O10 SO3 N 2O 4 Write Chemical Formula from Name: Work Backwords: Dinitrogen trioxide Diphosphorus pentoxide Assignments Pg 44, 45, 46. Naming Acids Naming acids that are binary: These would be covalent molecules that contain a hydrogen and another molecule (Usually a halogen or sulpfur or phosphorus) H__ Hydro______ic Acid Examples HI Hydrochloric acid Naming Acids Naming Acids that are polyatomic: These are covalent molecules containing hydrogen and a polyatomic ion. First we need to point out a pattern that you may or may not have already recognized: Naming Acids There are many sets of polyatomic ions that are similar to each other where the only difference is the number of oxygen atoms. The ions with more oxygens get an “ate” ending and the ion with less oxygens gets an “ite” ending. Ex. Phosphate PO43- and Phosphite PO33Nitrate: NO3- and Nitrite: NO2- Naming Acids The Oxygen containing polyatomic ions are named as follows: Least Oxygens: hypo_____ite ______ite ______ate Most Oxygens: per_______ate These are the polyatomic ions that are involved in acids Naming Acids When naming acids containing polyatomic ions we name them first by writing the name of the ion. We change the ending. If the ion ends in -ate we put an –“ic acid” on it. If the ion ends in ite we put an –“ous acid” ending on it. Examples Ex. H3PO3 ______________________ is the polyatomic ion in this acid, so the name is ______________________. Ex. HClO3: ClO3 is ______________________. Since it has an ate ending we name it ______________________. Examples Ex. Iodic acid. This has an ic ending so the ion must have an ____________ ending. Iodate (IO3-). Then we add however many ___________________we need to make the molecule neutral. So this molecule needs ______________________ hydrogens. ______________________ (We write the hydrogen first, then the polyatomic ion) Ex. Chlorous acid. This has an ous ending so we are looking for an ion with an _________ (with chlorine and oxygen). Chlorite (ClO2-). We add however many hydrogens we need. HClO2 Important: Note: On exams and assignments where naming is required, it is important that you are able to identify acids and name them appropriately. For example you name HCl hydrochloric acid, not hydrogen chloride. Assignment Page 47 Naming Organic IUPAC Naming System Organic compounds are those which contain carbon (excluding oxides and carbonates). Naming Organic Types of Hydrocarbons: Things to know for naming: Alkanes are organic compounds that contain only single bonds, Alkenes are carbon compounds that contain one or more double bonds Alkynes are organic compounds that contain one of more triple bonds. Naming Organic All Alkanes follow the CnH2n+2 rule. All Alkenes containing one double bond between carbons follow the CnH2n rule. These rules will help you to determine whether a molecule is an alkane, or an alkene. Naming Organic Ex. CH4 is an Alkane because if n=1, then the molecule would be C1H2(1)+2, Which is CH4. Is C2H6 and alkane or alkene? Is C2H2 an alkane or an alkene? Is C3H6 an alkane or alkene? Naming Organic Prefixes When naming organic compounds we must first find the longest chain of carbons in the molecule. Each number of carbons in the chains corresponds to a different prefix. Number of Carbons Prefix 6 Hex Eth 7 Hept 3 Prop 8 Oct 4 But 9 Non 5 Pent 10 Dec Number of Carbons Prefix 1 Meth 2 Naming Organic Suffix If the molecule contains all single bonds the suffix is “ane”. If the molecule contains a double bond the suffix is “ene”. If the molecule contains a triple bond the suffix is “yne”. Examples Alkenes and alkynes are named using a number to indicate where the double or triple bond is located. Always number the carbons so that you get the lowest possible number. Examples If the Compound contains 2 or more double bonds you would need to use di, tri etc. Ex. 1,2 butadiene or buta-1,2-diene Alkyl Groups If you remove a hydrogen atom from one of these carbon chains and add a branch, you get an alkyl group. These are also named based on the number of carbons but the suffix is “yl” For example methyl –CH3. These alkyl groups can then bond with another compound in place of a hydrogen. Alkyl Groups To name these we first identify the longest carbon chain. Next number the carbons. To write the name we first write the number, then the name of the alkyl group, then the name of the longest carbon chain. # - alkyl group longest carbon chain Alkyl Groups # - alkyl group longest carbon chain Ex 5. Alkyl Groups When naming compounds with more than one alkyl group, you must name them alphabetically. You also number the carbons so that the first group in the name has the lowest number. Alkyl Groups If you have two of the same alkyl group you name them as follows: #, # - dialkyl group longest carbon chain If you had three of the same alkyl groups you would use 3 numbers to indicate where they are located and the prefix tri on the name of the alkyl group. Alkyl Groups Alkyl Groups Ex 8. 2-methylpropane Ex 9. 3, 3- diethyl hexane Line Forms = Propane = 3-methyl pentane = Propene Draw the molecules from examples 1-9 in line form: