Name of Acid
advertisement

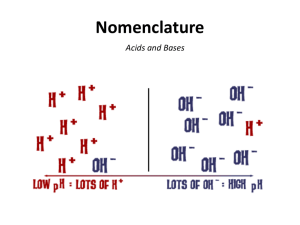
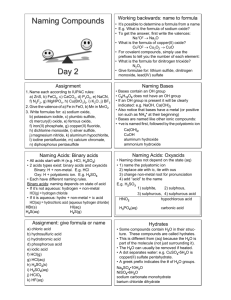
NAMIMG ACIDS First, know when you have an acid – Always start with H! *Look for this first when naming. There are 2 types of acids: Oxy acids and Binary acids 1) OXY ACIDS - They have “H” with a polyatomic ion (like SO42-) -> crisscross with H+ to get H2SO4 a) If the polyatomic ion bound to the H ends in –ate change the acid ending to –ic (see examples below). Example 1 - H2SO4 Example 2 - H3PO4 Example 3 - carbonic acid Sulphate -> sulphuric acid phosphate-> phosphoric acid H2CO3 b) If the polyatomic ion bound to the H ends in –ite change the acid ending to –ous (see examples below). Example 1 - H2SO3 Example 2 - H3PO3 Sulphite -> sulphurous acid Example 3 - carbonous acid phosphite-> phosphorous acid H2CO2 2) BINARY ACIDS - contain only hydrogen and one other element, e.g. HF. Rules 1. Use hydro as the prefix. 2. Then add the stem name of the second element. 3. Add an ic ending Formula Name of Acid HCl (aq) Hydrochloric acid H2S (aq) Hydrosulphuric acid HF (aq) Hydrofluoric acid For all acids, the number of H atoms is equal to the charge on the ion it is bonding with (crisscross). Practice makes Perfect Formula HF Name hydroiodic acid H3P sulphuric acid chloric acid HNO3 H2CO3 hydrosulphuric acid HClO3 Naming BASES – Contain hydroxide (OH-) ions - same as for any other polyatomic ion but you should know that if it has a hydroxide group, it is a BASE! Examples Name Sodium hydroxide Formula NaOH Calcium hydroxide Ca(OH)2 iron (III) hydroxide Fe(OH)3 Practice, Practice Practice Name Formula aluminum hydroxide Cu(OH)2 RbOH Barium hydroxide NAMING AND FORMULAS: MOLECULAR COMPOUNDS - Molecular compounds form between 2 NONMETALS (they will both have a negative charge). - Naming molecular compounds is different from ionic/polyatomic compounds because a prefix is used to indicate the number of each atom present for each element You need to memorize the following prefixes: 1 mono - 2 Di 3 Tri 4 5 6 7 Tetra Penta Hexa Hepta 8 9 Octa Nona 10 Deca Note: do not use “mono” for the first element Example 1 - sulphur trioxide Example 2 - diphosphorus pentasulphide SO3 P2S5 Practice Questions carbon dioxide ____________________ phosphorus trichloride ___________________ sulphur hexaflouride _______________ nitrogen monoxide _____________________ Naming Molecular Compounds: Example 1 - N2O Example 2 – BrI = ___________________ = dinitrogen monoxide Example 3 - XeF4 = ___________________ Practice Questions 1. N2O4 __________________________ 4. SO2 ______________________________ 2. H2O ___________________________ 5. P2O5 _____________________________ 3. CF4 ___________________________ 6. NCl3 _____________________________