atoms, molecules and ions
advertisement

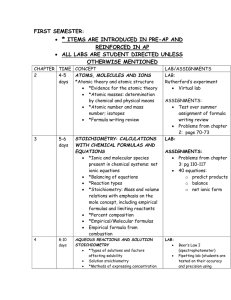
FIRST SEMESTER: ALL LABS ARE STUDENT DIRECTED UNLESS OTHERWISE MENTIONED CH. TIME CONCEPT LAB/ASSIGNMENTS 2 4-5 days ATOMS, MOLECULES AND IONS Atomic theory and atomic structure LAB: 3& 4 5-6 days Evidence for the atomic theory (p. 40-50) Atomic masses: determination by chemical and physical means (p. 77-80) Atomic number and mass number; isotopes (p. 50-52) Formula writing review (p. 52-54) STOICHIOMETRY: CALCULATIONS WITH CHEMICAL FORMULAS AND EQUATIONS 4 8-10 days Ionic and molecular species present in chemical systems: net ionic equations (p. 132-136, 145151) Balancing of equations (p. 99-102) Reaction types (p. 144) Stoichiometry: Mass and volume relations with emphasis on the mole concept, including empirical formulas and limiting reactants (p. 8197, 102-117) Percent composition (p. 88-90) Empirical/Molecular formulas (p. 90-97) Empirical formula from combustion (p. 90-92) AQUEOUS REACTIONS AND SOLUTION STOICHIOMETRY Types of solutions and factors affecting solubility (p. 130-136) Solution stoichiometry (p. 151-154) Methods of expressing concentration (p. 136144) o Molarity o Dilution o Mixing of solutions o Electrolytic properties Weak, strong and non electrolytes Ionic and molecular species present in chemical systems (p. 132-136, 145-151) Net ionic equations (p. 132-136, 145-151) Atom board practice of atomic and mass numbers and isotopes ASSIGNMENTS: Formula writing review Problems from chapter 2 LAB: Analysis of a Penny and Chemical Reactions and Equations ASSIGNMENTS: Problems from chapter 3: pg 110-117 40 equations: o predict products o balance o net ionic form LAB: Conductivity of Aqueous Solutions Precipitating Calcium Phosphate ASSIGNMENTS: Chapter 3 problems Worksheet on combining solutions and calculating ions in solution. 6 14-16 days THERMOCHEMISTRY State functions (p. 236-242) First law: change in enthalpy; heat of formation; heat of reaction; Hess’s law, heats of vaporization and fusion; calorimetry (p. 243-262) LAB: Energy and Specific Heat Thermochemistry and Hess’s law ASSIGNMENTS: 12 11 days CHEMICAL KINETICS Concept of rate of reaction (p. 540-547) Use of experimental data and graphical analysis to determine reactant order, rate constants and reaction rate laws (p. 547-562) Effect of temperature change on rates (p. 565570) 13 11 days Energy of activation; the role of catalysts (p. 565-577) The relationship between the rate-determining step and a mechanism (p. 562-565) Half-lifes (p. 554-561) CHEMICAL EQUILIBRIUM Concept of dynamic equilibrium, physical and chemical, LeChatelier’s principle; equilibrium constants (p. 594-601, 620-626) Quantitative treatment o Equilibrium constants for gaseous reactions: Kp,Kc (p. 601-620) Book problems LAB: Factors affecting Rate (kit from FLINN) Crystal violet Lab with CBL o Determines order and activation energy ASSIGNMENTS: Book problems Determine order of reaction through graphical analysis LAB: LeChatelier principle (kit) SECOND SEMESTER 4, 14, & 15 11 days ACID-BASE EQUILIBRIA (p. 154) Equilibrium constants for reactions in solution (p. 640-644) Constants for acids and bases: pK; pH (p. 647) Bronsted-Lowry Acids and Bases (p. 639-642) The auto-ionization of water (p. 645) PH scale (p. 647) Indicators (p. 728-733) Strong acids & bases compared to weak acids and weak bases (p. 650-657, 661-666) Relationship between Ka and Kb (p. 663-666) Acid-Base properties of salt solutions (p. 671677) LAB: Calculation of Ka of an acid (indicator) 15 & 16 25 days Acid-Base behavior and chemical structure (p. 639-642) Triprotic acids (p. 666-671) Lewis acids and bases (p. 679-682) ADDITIONAL ASPECTS OF AQUEOUS EQUILIBRIA Common ion effect; buffers; hydrolysis (p. 671677, 698-713) Acid-base titration (p. 157-160. 713-727) Solubility equilibria; solubility product constants and their application to precipitation and the dissolution of slightly soluble compounds (p. 744-759) Coordination complexes; amphoterism (p. 759764) Precipitation reactions (p. 145-151) Qualitative analysis for metallic elements (p. 757-759) 17 & 18 7 days CHEMICAL THERMODYNAMICS Second law: entropy; free energy of formation; free energy of reaction (p. 773-798) Dependence of change in free energy on enthalpy and entropy changes and temperature changes (p. 773-798) Relationship of change in free energy to equilibrium constants and electrode potentials (p. 798-802, 833-842) 4& 18 17 days ELECTROCHEMISTRY Oxidation-reduction reactions; balancing equations (p. 161, 166-168) Oxidation number (p. 162-165) The role of the electron in oxidation-reduction (p. 165-166) Electrochemistry’ electrolytic and galvanic cells; Faraday’s Lasw; standard half-cell potentials; Nernst equation; prediction of the direction of redox reactions (p. 823-836, 838-842, 847-851) Cell EMF under standard conditions (p. 833836) Free energy and redox reactions (p. 825-833) Cell EMF under nonstandard conditions (p. 836842) Electrolysis (p. 847-851) 7 3 days ELECTRONIC STRUCTURE OF ATOMS Electron energy levels (p. 285-309) Atomic spectra (p. 312-318) Quantum numbers (p. 300-304) Atomic orbitals (p. 305) LAB: Titration of strong acid and strong base; standardization of a base Determination of molecular mass of a weak acid through titration Construction of a pH curve through titration Descriptive/qualitative analysis determination of unknown salts DEMO: LABS: The Potential of Electrochemical Cells(Salt bridge) Electrolysis of KI solution 7& 8 3-5 days PERIODIC PROPERTIES 2, 8, 9, & 10 5 days 5 days 5-8 days Binding forces (p. 52-54, 341-344, 357-361, 440-461) o Ionic o Covalent o Metallic hydrogen bonding o Van der Waals o London dispersion forces Relationships to states, structure, and properties of matter (p. 466-478) Polarity of bonds (p. 346-349) Electronegativity (p. 344-346) Resonance structures (p. 373-378) Exceptions to the octet rule (p. 369-373) Strengths of covalent bonds o Bond energy (p. 341, 353-357, 361-364) o Bond length (p. 342, 422-423, 362-363) MOLECULAR GEOMETRY AND BONDING THEORIES 1, 8 10, & 11 Relationships in periodic table (p. 318-320, 323327) atomic & ionic radii, ionization energies, electron affinities, oxidation states, electronegativity (p. 309-311, 320-323, 344-346, 353) effective nuclear charge, shielding affect, metallic character (p. 308-309, 320) BASIC CONCEPTS OF CHEMICAL BONDING 8, 9, & 10 Aufbau, Pauli and Hund’s Rule (p. 306-308, 312-318) Lewis structures (p. 365-373) Valence Bond: hybridization of orbitals, resonance, sigma and pi bonds (p. 373-378, 404-416) VSEPR (p. 378-390) Geometry of molecules and ions, structural isomerism of molecules and coordination complexes, dipole moments and relation of properties to structure (p. 346-349, 378-390, 440-443, 969-974) INTERMOLECULAR FORCES, LIQUIDS AND SOLIDS LAB: States of matter (p. 26) Molecular geometry structures with balls and sticks and Styrofoam for the expanded octets. LAB: Triple point of dry ice Molecular mass 11 & 16 5-8 days PROPERTIES OF SOLUTIONS 1, 2, &5 19 5 days 2-3 days Liquids and solids from the kinetic-molecular viewpoint (p. 439-440, 565-570) Phase diagrams of one-component systems (p. 479-483) Changes of state, including critical points and triple points (p. 475-479) Structure of solids; lattice energies (p. 353-357, 445-471) Solution process (p. 498-504) Saturated solutions and solubility (p. 744-752) Factors affecting solubility (p. 504-509) Ways of expressing concentration (p. 136-144, 498-501) Colligative properties (p. 516-519, 524-527) Raoult’s Law (p. 509-515) Nonvolatile compared to volatile solutes (p. 509515) Osmosis (p. 520-524) Nonideal behavior and quantitative methods (p. 513, 525) Ideal solutions (p. 514-515) GASES Characteristics of Gases (p. 26) Gas Laws of ideal gases: Charles, Boyles, Grahams, Combined Gas Law, Daltons Law of Partial Pressures, Guy Lussac, and Graham’s Law of Effusion (p. 183-194, 199-205, 212-214) Equation of State of an Ideal Gas (p. 190-191) Kinetic molecular theory of gases (p. 205-212) o Interpretation of ideal gas laws on the basis of kinetic theory Molecular effusion and diffusion (p. 212-214) Real Gases: deviations from ideal behavior (p. 214-217) Dependence of kinetic energy of molecules on temperature (p. 206, 210) Avogadro’s hypothesis and the mole concept (p. 45-46, 208) NUCLEAR CHEMISTRY (p. 873-894) Nuclear equations Half-lives Radioactivity Chemical applications determination using freezing and boiling points Evaporation and Intermolecular Attractions LAB: Using Conductivity to Find an Equivalence Point Vapor Pressure of Liquids LABS: Determination of molecular mass from a gas (butane)