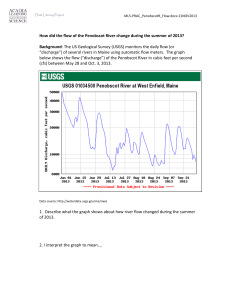
New developments in research on ME/CFS
advertisement

2015 Webinar Series | Thursday, October 15, 2015 | 1:00 PM Eastern New Developments in ME/CFS Research Alan R. Light, PhD Research Professor of Anesthesiology University of Utah www.SolveCFS.org About Our Webinars • Welcome to the 2015 webinar series! • The audience is muted; use the question box to send us questions • Webinars are recorded, and the recording is made available on our YouTube channel • SMCI is a research organization and does not provide medical advice 2015 Webinar Series | Thursday, October 15, 2015 | 1:00 PM Eastern New Developments in ME/CFS Research Alan R. Light, PhD Research Professor of Anesthesiology University of Utah www.SolveCFS.org “New developments in research on ME/CFS” Alan R. Light, Ph.D. Depts. Anesthesiology, and Neurobiology and Anatomy: University of Utah School of Medicine Ron Hughen (does everything) Kathy Light (human research) Andrea White (exercise scientist) Cindy Bateman, M.D. (CFS clinician extraordinaire) Jie (Jesse) Zhang Chris Benson, M.D. Markus Amann Supported by grants from: Dept. of Anesthesiology U of U School of Medicine U of U NINDS NIHLB SolveCFS AFSA Recent Advances in ME/CFS Research • Three new publications indicate that autoimmune disease may cause CFS in a subgroup of patients. • Two of these papers suggest that it might be treatable in some of these patients “New developments in research on ME/CFS” • #1. Autoimmune Basis for Postural Tachycardia Syndrome • Hongliang Li, Xichun Yu, Campbell Liles, Muneer Khan, Megan Vanderlinde-Wood, Allison Galloway, Caitlin Zillner, Alexandria Benbrook, Sean Reim, Daniel Collier, Michael A. Hill, SatishR. Raj, Luis E. Okamoto, Madeleine W. Cunningham, Christopher E. Aston and David C. Kem J Am Heart Assoc.2014; 3: e000755originally published February 26, 2014 • Postural Tachycardia Syndrome is common in a subset of patients with ME/CFS • It involves a rapid increase in heart rate when standing up • Unlike other forms of orthostatic intolerance where blood pressure remains low or decreases with standing, blood pressure can actually increase with standing • This investigation looked at 14 patients with POTS (postural orthostatic tachycardia syndrome) and 10 healthy subjects. • They initially did the study unblinded on 7 POTS patients and 8 controls. Then used blinded samples from Vanderbilt University to look at 7 more POTS patients and 2 controls. “New developments in research on ME/CFS” • Autoimmune Basis for Postural Tachycardia Syndrome • Briefly, this report indicates that in patients with POTS, a condition that many CFS patients have, most if not all of their symptoms may be caused by auto antibodies against components of the cardiovascular system “New developments in research on ME/CFS” Beta adrenergic receptor gain of function autoantibodies are present in POTS patients Increase in beta 1 activity would cause the increased heart rate in these patients, increase in beta 2 activity would decrease blood pressure OKLA VANDER OKLA VANDER “New developments in research on ME/CFS” Alpha Adrenergic receptor loss of function autoantibodies are present in POTS patients This would cause loss of normal vasoconstriction in POTS patients OKLA OKLA VANDER VANDER “New developments in research on ME/CFS” • #2. The Norwegian Group published the results of a new trial in July 2015. • B-Lymphocyte Depletion in Myalgic Encephalopathy/ Chronic Fatigue Syndrome. An Open-Label Phase II Study with Rituximab Maintenance Treatment. Fluge Ø, Risa K, Lunde S, Alme K, Rekeland IG, Sapkota D, et al. (2015) PLoS ONE 10(7): e0129898. • In 2009, this same group had done a case series that indicated positive effects of Rituximab in some patients with CFS “New developments in research on ME/CFS” • In 2011, this this group had done a small, randomized, double-blind and placebocontrolled phase II study of 30 patients given either rituximab (two infusions two weeks apart), or placebo, with follow-up for 12 months. • The primary endpoint was negative. There was no difference between the rituximab and placebo groups at 3 months follow-up. • However, they had seen some evidence during later followups that Fatigue scores improved between 6–10 months after the treatment with clinical responses in 2/3 of the patients receiving rituximab “New developments in research on ME/CFS” • The new study is a follow up based on the longterm findings in their previous study • The new study was an open-label, study with 29 patients. • They were treated with rituximab - two infusions two weeks apart, followed by maintenance rituximab infusions after 3, 6, 10 and 15 months, and with follow-up for 36 months. • Results showed: • lasting improvements in self-reported Fatigue score, in 18 out of 29 patients “New developments in research on ME/CFS” • At end of follow-up (36 months), 11 out of 18 responding patients were still in ongoing clinical remission. • For major responders, the mean lag time from first rituximab infusion until start of clinical response was 23 weeks (range 8–66). • Conclusions: The observed patterns of delayed responses and relapse after B-cell depletion and regeneration, a three times higher disease prevalence in women than in men, and a previously demonstrated increase in B cell lymphoma risk for elderly ME/CFS patients, suggest that ME/CFS may be a variant of an autoimmune disease. “New developments in research on ME/CFS” • #3. Antibodies to ß adrenergic and muscarinic cholinergic receptors in patients with Chronic Fatigue Syndrome • Madlen Loebel, Patricia Grabowski, Harald Heidecke, Sandra Bauer, Leif G. Hanitsch,Kirsten Wittke, Christian Meisel, Petra Reinke, Hans-Dieter Volk, Oystein Fluge, OlavMella, Carmen Scheibenbogen. Brain, Behavior, and Immunity (October 2015) • This was a collaboration between a German group who are experts in Elisa for autoantibodies and the previous Norwegian group who did the Rituximab treatment trial “New developments in research on ME/CFS” • Approximately 30% of the 268 patients with CFS had autoantibodies against beta 2 adrenergic receptors and or acetylcholine receptors • Responders to B cell reduction therapy from the previous trial (Rituximab) included 15 out of 25 treated CFS patients, while nonresponders were 10 of the 25. • Many of the responders had high levels of autoantibodies against beta adrenergic receptors or acetylcholine receptors. • The levels of these autoantibodies decreased to normal levels following successful treatment with Rituximab. “New developments in research on ME/CFS” “New developments in research on ME/CFS” • These reports, and our own published gene expression studies in which 30% of patients with ME/CFS showed a large decrease in adrenergic alpha receptors following exercise and had orthostatic intolerance convinced us to look for auto antibodies in this specific group of CFS patients. • Currently, Dr. Madeline Cunningham and Dr. Kem are running blinded autoantibody assays on serum from CFS patients we provided to them. “New developments in research on ME/CFS” • We recently conducted a double blind, cross over trial of pregabalin (Lyrica) for Fibromyalgia and Chronic Fatigue Syndromes. • We did this trial because our gene expression studies indicated that pregabalin and gabapentin were effective in normalizing gene expression and decreasing pain and fatigue in a subset of our patents. • Disclosure: This was an Investigator Initiated Grant from Pfizer The results can and will be published freely, after allowing Pfizer a prior look at the manuscript. • • Treatment Protocol We examined pre- and post-exercise leukocyte gene expression changes induced by pregabalin in 10 patients with FM alone and 10 patients with both CFS and FM. • In each of the 2 patient groups, 5 patients were randomly assigned to receive pregabalin as Treatment 1, and 5 others received pregabalin as Treatment 2. • Initial total daily doses were 150 mg, then titrated upward over 2 weeks to achieve 300-450 mg, then maintained at the highest effective and tolerable dose for 3 additional weeks. Results All Patients N=20 FM only N=9 CFS+FM N=11 2 male, 18 female 1 male, 8 female 1 male, 10 female Responders, N=13 6 CFS+FM, 7FM Non-Responders, N=7 5 CFS+FM, 2 FM 1 male, 12 female 1 male, 6 female In responders, large improvement in symptoms: 24 points for pain, 19 points for physical fatigue, 15 points for mental fatigue, 100 100 100 9090 90 80 80 70 70 60 60 50 50 40 40 30 30 20 20 20 10 10 10 0 00 Physical Mental Pain Fatigue Fatigue on Lyrica on Lyrica on Placebo on Placebo on Lyrica Pain MFbase PFbase base Pain MFmid PFmid mid Pain MFimm PFimm imm on Placebo Pain MF30 PF30 30 min Pain MF8 PF8 8 hr Pain MF24 PF24 24 hr Pain MF48 PF48 48hr Heart rate is decreased during exercise for most patients who respond to Lyrica Even though these patients actually do more work during the exercise when on Lyrica. Heart rate for Lyrica Responders on Placebo vs on Lyrica Placebo Lyrica Heart rate for Lyrica Non-Responders on Placebo vs on Lyrica 160 160 150 150 140 140 130 130 120 120 110 110 100 100 90 80 Placebo Lyrica 90 80 Gene Expression Changes with Lyrica ASIC3 This graph compares the gene expression changes caused by exercise in controls vs. patients P2X4 1.5 Non-responding patients onon Lyrica Lyrica Responding Patients placebo Non-responding Lyrica Responding Patients Patients on on Placebo Lyrica TRPV1 1.4 COMT * * 1.3 TLR4 P2X7 1.2 TNFbeta * 1.1 1 baseline baseline 0.9 * 0.8 8 hr 0.7 24 hr 48 hr 8 hr 24 hr 48 hr Conclusions • Pregabalin can be at least a short term treatment for a subset of CFS patients as well as for a subset of FMS patients • Some patients can have a worsening of symptoms when given pregabalin • Physiological measures are associated with behavioral improvement in CFS symptoms caused by pregabalin Be at the Center of the Solution Register for additional webinars: SolveCFS.org/webinar Sign up for our blog and newsletters SolveCFS.org/newsletters Like us on Facebook.com/CFIDSAssn Join the BioBank! Email BioBank@SolveCFS.org SolveCFS.org/biobank Drive research and fuel progress by donating! SolveCFS.org/donate Thank You! Our Mission Make ME/CFS understood, diagnosable and treatable. Our Strategy Stimulate participatory research aimed at the early detection, objective diagnosis and effective treatment of ME/CFS through expanded public, private and commercial investment