Chapter #1
advertisement

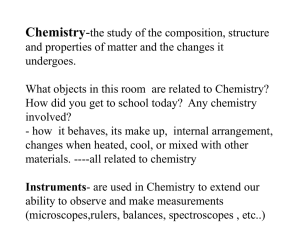
Chapter 1- matter and change Bravo – 15,000 kilotons Do Not Need in your Notebook Introduction • “It has been said that Chemistry is what connects us to the world. Everything we see, taste, and touch is matter. Matter is what makes the world a real place. Chemistry is the study of matter: its properties, composition, and how some types of matter interact with other types of matter in new and interesting combinations. • Chemistry is heavily involved in many interesting and fascinating jobs, from making perfumes to investigating crime scenes, from preparing great tasting and safe foods to developing life-saving drugs.” Section 1-1 • Chemistry a physical science, is the study of the composition, structure, and properties of matter and the changes it undergoes. • Chemical is any substance that has a definite composition. Example water is always H2O. Everything is made of chemicals. • Branches of chemistry. Chemistry is divided into six main areas of study. 6 Branches of Chem 1. Organic Chemistry- The study of most carbon containing compounds. Example C6H12O6 Glucose 2. Inorganic Chemistry- The study of all substances not classified as organic; compounds that do not contain carbon. Example H2O Water. High school chemistry is mostly inorganic chemistry. 3. Physical Chemistry (P-Chem)- the study of the properties, changes, and relationship between energy and matter. 4. Analytical chemistry- the identification of the components and composition of materials. 5. Biochemistry- the study of substances and processes occurring in living things. Example Photosynthesis 6. Theoretical Chemistry- the use of mathematics and computers to design and predict the properties of new compounds. Types of Research 1. Basic Research-how and why 2. Applied Research- solve a specific problem. Example the depletion of the ozone layer. 3. Technological Development-involves the production and use of products that improve our quality of life. Technology is the application of scientific knowledge to solve problems. Examples Computers and biodegradable materials. Section 1-2 • Mass is the measure of the amount of matter. • Matter is anything that has mass and volume (occupies) space. Examples air, smoke, water vapor. • Atom is the smallest unit of an element that maintains the properties of that element. • Element is a pure substance made of only one kind of atom. Periodic Table. • Compound is a substance that is made from the atoms of two or more elements that are chemically bonded. • Physical properties can be observed or measured without altering the identity of a material. Example melting ice to find the melting point. • Extensive Physical Properties- depend on the amount of matter present and include mass, length, and volume. • Intensive Physical Properties- do NOT depend of the amount of matter. Examples melting point, boiling point, Density, ductility, malleability, color, crystal shape • Physical change is any change that does NOT result in a change in identity. Examples cutting wire, crushing a solid, gas expanding. • Changes in state is a physical change. Example melting, boiling, freezing. • Chemical Properties relates to a substances ability to undergo changes that alters it identity. Example a chemicals reactivity. • Chemical change or reaction (Rxn) when a substance is converted into different substance. Examples milk souring, leaves changing color in the fall. • Basic Chemical Formula • A+BC • A and B are called Reactants • is read as “yields” • C is the product (s) 4 states of matter 1. Solids have definite shape and volume. Particles are packed closely together. 2. Liquids have definite volume but, no shape 3. Gases have neither a definite volume nor definite shape. 4. Plasma is a gaseous system of charges particles. Found on the sun. Weight vs. Mass • Weight is the pull of gravity on an object. • Mass is the quantity of matter. • Astronauts in space. • Law of conservation of mass states that matter can NOT be either created or destroyed by ordinary chemical or physical changes. Examples burning wood, nuclear reactions • Law of conservation of energy states that E can NOT be either created or destroyed but it can be converted from one form to another. Examples Kinetic & potential energy. Classification of Matter • Mixture is a blend of two or more kinds of matter, each of which retains its own identity and properties and can be separated physically. • 2 types of mixtures 1. Homogeneous (solutions) have uniform composition throughout. Examples air, sugar in water, stainless steel. 2. Heterogeneous not uniform. Examples granite, wood, blood. Do Not Need in your Notebook Matter Can it be separated? YES NO Mixtures Pure Substances Is the composition uniform? Can it be decomposed by ordinary chemical means? YES NO YES NO Homogenous Mixtures Air, sugar in water, stainless steel Heterogeneous Mixtures Granite, wood, blood Compounds Water, sodium chloride, sucrose Elements Gold, aluminum, oxygen o A pure substance has a fixed composition and differs from a mixture by all pure substance have exactly the same physical and chemical properties and every sample of given pure substance has exactly the same composition. o Pure substances can be classified as either compounds or elements. Pure substances • Compound is a substance that is made from the atoms of two or more elements that are chemically bonded. Examples water, sodium chloride, sucrose. Can not be separated physically. • Element made of one pure substance that cannot be decomposed by chemical changes. Examples gold, silver, hydrogen. Section 1-3 • Periodic table of elements is divided into small squares that have one element in each square. • Elements have been named from their Latin meaning, places, famous scientist and from mythology. Nonmetals F A M I L Metals Lf of step Period N O B L E y • Family or groups- vertical columns (18) • Period- horizontal rows (7) • Metals- ductile, malleable, lustrous, conduct heat and electricity, high tensile strength • Nonmetals- brittle, dull, poor conductor • Metalloids- have some characteristics of metals and nonmetals. Are semiconductors • Nobel gases- family #18 unreactive gases as room temp. WHY??? Work Cited • July 25, 2006. “Pigs in Space”. JPG. http://www.porphyrios.it/index.php?m=20050718 • July 25, 2006. “Chemistry”. http://www.aprenda.info/Chemistry.asp • July 27, 2006. “Periodic Table illustration”. http://www.physast.uga.edu/classes/phys1010/heil/announc ement%20files/equations/unit_4_eqs/periodic_table_illust r.png • Holt, Rinehart and Winston. Modern Chemistry. Harcourt Brace & Company. 1999. • Title slide “Bomb picture”. July 25, 2006. http://www.sciencegeek.net/Chemistry/Powerpoint/Unit1/U nit1_files/frame.htm